ICATIBANTO CIPLA 30 mg Injectable Solution in Pre-filled Syringe

How to use ICATIBANTO CIPLA 30 mg Injectable Solution in Pre-filled Syringe
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Icatibant Cipla 30 mg Solution for Injection in Pre-filled Syringe EFG
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Icatibant Cipla and what is it used for
- What you need to know before you use Icatibant Cipla
- How to use Icatibant Cipla
- Possible side effects
- Storage of Icatibant Cipla
- Contents of the pack and other information
1. What is Icatibant Cipla and what is it used for
Icatibant Cipla contains the active substance icatibant.
This medicine is used to treat the symptoms of hereditary angioedema (HAE) in adults, adolescents, and children over 2 years of age.
In HAE, the levels of a substance in the blood called bradykinin increase, producing symptoms such as swelling, pain, nausea, and diarrhea.
Icatibant blocks the action of bradykinin and thus slows down the progression of the symptoms of an HAE attack.
2. What you need to know before you use Icatibant Cipla
Do not use Icatibant Cipla
- if you are allergic to icatibant or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor before starting to use Icatibant Cipla:
- If you have angina pectoris (reduced blood flow to the heart).
- If you have recently had a stroke.
The side effects related to icatibant are similar to the symptoms of your own illness. Consult your doctor immediately if you notice that the symptoms of the attack worsen after you are given this medicine.
In addition:
- You or your caregiver must learn the technique for subcutaneous injections (under the skin) before you self-administer or your caregiver administers this medicine to you.
- Immediately after self-administering Icatibant Cipla or after your caregiver administers it to you while you are experiencing a laryngeal attack (obstruction of the upper airway), you should seek medical attention at a medical institution.
- If your symptoms do not resolve after a self-administered injection of Icatibant Cipla or after an injection administered by your caregiver, you should consult your doctor about the administration of additional injections of this medicine. In adult patients, up to 2 additional injections can be administered within 24 hours.
Children and adolescents
This medicine is not recommended for use in children under 2 years of age or weighing less than 12 kg because it has not been studied in these patients.
Other medicines and Icatibant Cipla
Tell your doctor if you are taking, have recently taken, or might take any other medicines.
No interactions of Icatibant Cipla with other medicines are known. If you are taking any medicine that is an angiotensin-converting enzyme inhibitor (ACEI) (e.g., captopril, enalapril, ramipril, quinapril, lisinopril) to lower blood pressure or for any other reason, inform your doctor before using this medicine.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before taking this medicine.
If you are breastfeeding, you should not breastfeed your child for 12 hours after the last administration of this medicine.
Driving and using machines
Do not drive or use machines if you feel tired or dizzy as a result of the HAE attack or after using this medicine.
Icatibant Cipla contains sodium
This medicine contains less than 1 mmol (23 milligrams) of sodium per syringe, so it is essentially "sodium-free".
3. How to use Icatibant Cipla
Follow the instructions for administration of this medicine exactly as indicated by your doctor. In case of doubt, consult your doctor again.
If you have never been administered Icatibant Cipla before, the first dose should always be injected by medical or nursing staff. Your doctor will discharge you when they consider it safe for you to go home.
After discussing with your doctor or nurse and learning the technique for subcutaneous injections (under the skin), you or your caregiver can administer this medicine to you if you have an HAE attack.
It is essential to inject Icatibant Cipla subcutaneously (under the skin) as soon as you notice an angioedema attack. The healthcare staff will teach you and your caregiver how to inject this medicine safely, following the instructions in the package leaflet.
When and how often should you use Icatibant Cipla?
Your doctor has determined the exact dose of this medicine and will tell you how often you should use it.
Adults
- The recommended dose of Icatibant Cipla is one injection (3 ml, 30 mg) administered subcutaneously (under the skin) as soon as you notice the angioedema attack (e.g., with increased skin swelling, especially on the face and neck, or increased abdominal pain).
- If you do not notice an improvement in symptoms after 6 hours, you should seek medical advice about the administration of additional injections of Icatibant Cipla. In adults, up to 2 additional injections can be administered within 24 hours.
- You should not receive more than 3 injections in a 24-hour period, and if you need more than 8 injections in a month, consult your doctor.
Children and adolescents from 2 to 17 years
- The recommended dose of this medicine is an injection of 1 ml up to a maximum of 3 ml, depending on body weight, administered subcutaneously (under the skin) as soon as symptoms of an angioedema attack appear (e.g., increased skin swelling, especially on the face and neck, or increased abdominal pain).
- Consult the section on instructions for use to see the dose you should inject.
- If you are unsure about the dose to inject, consult your doctor, pharmacist, or nurse.
- If your symptoms worsen or do not improve, you should seek medical advice immediately.
How should Icatibant Cipla be administered?
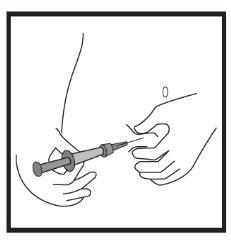
Icatibant Cipla is administered by subcutaneous injection (under the skin). Each syringe should only be used once.
This medicine is injected with a short needle into the fatty tissue under the skin of the abdomen (belly).
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
The following step-by-step instructions are intended for:
- self-administration (adults)
- administration by a caregiver or healthcare professional for adults, adolescents, or children over 2 years of age (weighing at least 12 kg).
The instructions include the following main steps:
- General information
2a) Preparation of the syringe for children and adolescents (2-17 years) weighing 65 kg or less
2b) Preparation of the syringe and needle for injection (all patients)
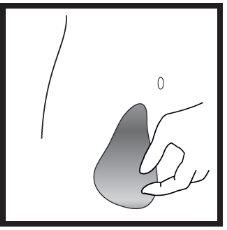
- Preparation of the injection site
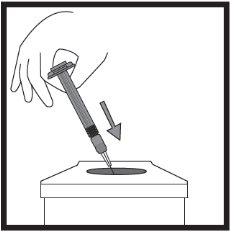
- Injection of the solution
- Disposal of injection materials
Step-by-step instructions for injection
| ||||||||||
| ||||||||||
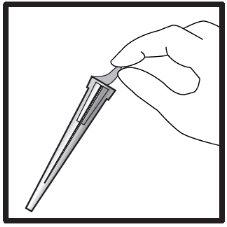
2a) Preparation of the syringe for children and adolescents (2-17 years) weighing 65 kg or less: | ||||||||||
Important information for healthcare professionals and caregivers: When the dose is less than 30 mg (3 ml), the following equipment is needed to extract the correct dose (see information below):
The required injection volume in ml should be prepared in an empty 3 ml graduated syringe (see table below). Table 1: Dosage guideline for children and adolescents
Patients weighing more than 65 kgwill use the entire contents of the pre-filled syringe (3 ml).
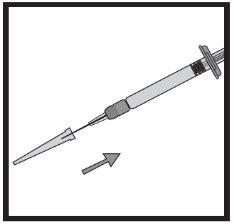
Transfer the icatibant solution to the graduated syringe:
| ||||||||||
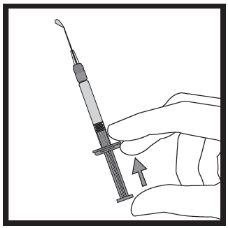
If there is air in the graduated syringe:
| ||||||||||
|
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. Almost all patients who receive Icatibant Cipla notice a reaction at the injection site (such as skin irritation, inflammation, pain, itching, redness of the skin, and burning). These effects are usually mild and improve without the need for any additional treatment.
Tell your doctor immediately if you notice that the symptoms of the attack worsen after you have received this medicine.
Very common(may affect more than 1 in 10 people):
- Additional reactions at the injection site (feeling of pressure, bruising, decreased sensitivity and/or numbness, increased skin rash with itching and heat).
Common(may affect up to 1 in 10 people):
- Nausea
- Headache
- Dizziness
- Fever
- Itching
- Rash
- Redness of the skin
- Abnormal liver function tests
Frequency not known(cannot be estimated from the available data):
- Hives (urticaria)
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Icatibant Cipla
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton after "EXP". The expiry date refers to the last day of the month shown.
Do not store above 25°C. Do not freeze.
Do not use this medicine if you notice that the syringe or needle packaging is damaged or if you notice visible signs of deterioration; for example, if the solution is cloudy, contains floating particles, or has changed color.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Content and Additional Information
Composition of Icatibant Cipla
- The active ingredient is icatibant. Each pre-filled syringe contains 30 milligrams of icatibant (as acetate). Each ml of solution contains 10 mg of icatibant
- The other components are sodium chloride, glacial acetic acid, sodium hydroxide (for pH adjustment) and water (see section 2).
Appearance of the Product and Container Content
Icatibant Cipla is presented as a clear and colorless injectable solution in a 3 ml glass pre-filled syringe. The container contains a hypodermic needle.
Icatibant Cipla is available in a single pack of one pre-filled syringe with one needle or in a multipack of three pre-filled syringes with three needles.
Only some pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Cipla Europe NV
De Keyserlei 58-60, Box 19,
2018 Antwerp, Belgium
Manufacturer
Pharmadox Healthcare Ltd.
KW20A Kordin Industrial Park
Paola PLA 3000
Malta
or
Eurofins PROXY Laboratories (PRX)
Archimedesweg 25 2333 CM Leiden
Netherlands
Local Representative
Cipla Europe NV branch in Spain,
C/Guzmán el Bueno, 133 Edif Britannia 28003 Madrid, Spain
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Germany | Icatibant Cipla 30 mg Injection Solution i.e. Prefilled Syringe |
Denmark | Icatibant Cipla |
Spain | Icatibanto Cipla 30 mg injectable solution in pre-filled syringe EFG |
Norway | Icatibant Cipla |
Date of the Last Revision of this Leaflet:September 2021
Detailed and updated information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/)
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ICATIBANTO CIPLA 30 mg Injectable Solution in Pre-filled SyringeDosage form: INJECTABLE, 30 mgActive substance: icatibantManufacturer: Takeda Pharmaceuticals International Ag Ireland BranchPrescription requiredDosage form: INJECTABLE, 30 mgActive substance: icatibantManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: INJECTABLE, 30 mgActive substance: icatibantManufacturer: Laboratoire AguettantPrescription required
Online doctors for ICATIBANTO CIPLA 30 mg Injectable Solution in Pre-filled Syringe
Discuss questions about ICATIBANTO CIPLA 30 mg Injectable Solution in Pre-filled Syringe, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions





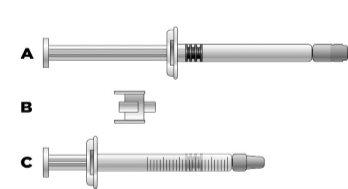
 If you are unsure about the volume of solution to extract, consult your doctor, pharmacist, or nurse
If you are unsure about the volume of solution to extract, consult your doctor, pharmacist, or nurse Avoid touching the ends of the connector and the tips of the syringe to avoid contamination.
Avoid touching the ends of the connector and the tips of the syringe to avoid contamination.