
GRAZAX 75000 SQ-T SUBLINGUAL LYOPHILIZED


How to use GRAZAX 75000 SQ-T SUBLINGUAL LYOPHILIZED
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Grazax 75,000 SQ-T Sublingual Lyophilisate
Standardized allergen extract of grass pollen
Phleum pratense(Timothy grass)
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
-Keep this leaflet, you may need to read it again.-If you have any further questions, ask your doctor or pharmacist.
-This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
-If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What is Grazax and what is it used for
- What you need to know before you start taking Grazax
- How to take Grazax
- Possible side effects
- Storage of Grazax
- Contents of the pack and further information
1. What is Grazax and what is it used for
Grazax contains a grass pollen allergen extract. Grazax is used for the treatment of rhinitis and conjunctivitis caused by grass pollen in adults and children (5 years or older). Grazax modifies the allergic disease by increasing immunological tolerance to grass pollen.
The selection of children to receive treatment should be made by doctors specializing in the treatment of allergic diseases in children.
Your doctor will assess your allergic symptoms and perform a skin diagnostic test or take a blood sample to decide if Grazax should be used as treatment.
It is recommended to take the first sublingual lyophilisate under medical supervision. This is a precaution to assess each patient's sensitivity to the treatment. This gives you the opportunity to discuss any possible side effects with your doctor.
Grazax is prescribed by doctors specializing in the treatment of allergies.
2. What you need to know before taking Grazax
Do not take Grazax:
- if you are allergic (hypersensitive) to any of the excipients of this medicine (listed in section 6).
- if you have a disease that affects the immune system.
- if you have severe asthma (diagnosed by your doctor).
- if you have cancer.
- if you have severe inflammation in the mouth.
Warnings and precautions:
Talk to your doctor before starting to take Grazax
- if you have recently had a tooth extraction or any other type of mouth surgery. In this case, you should interrupt treatment with Grazax for 7 days to allow complete healing of the mouth.
- if you have a severe fish allergy.
- if you have previously had an allergic reaction related to the injection of a grass pollen allergen extract.
- if you have asthma and have an acute upper respiratory tract infection. You should temporarily discontinue treatment with Grazax until the infection is resolved.
Some side effects can be serious and require immediate medical treatment. Please see section 4 for symptoms.
Children
- Loss of the first teeth (milk teeth). Treatment with Grazax should be interrupted for 7 days to allow complete healing of the mouth.
If you are in one of these situations, inform your doctor before starting to take Grazax.
There is no experience with Grazax in the elderly (65 years or older).
Other medicines and Grazax
Tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including those obtained without a prescription. If you are taking other medicines to treat allergy symptoms, such as antihistamines or corticosteroids, your doctor should assess the use of these medicines.
Taking Grazax with food and drinks
You should wait 5 minutes after taking the sublingual lyophilisate before consuming food and/or drinks.
Pregnancy, breastfeeding, and fertility
There is currently no experience with the use of Grazax during pregnancy. Treatment with Grazax should not be started during pregnancy. If you become pregnant during treatment, consult your doctor about the risks of continuing treatment.
There is currently no experience with the use of Grazax during breastfeeding. No effects on breastfed infants are expected.
Driving and using machines
You are the only person responsible for judging your ability to drive or perform precision work. The effects or side effects of the medicine may affect this ability. These effects are described in other parts of this leaflet. Therefore, read the entire leaflet for your information.
Talk to your doctor or pharmacist if you have any doubts.
The influence of treatment with Grazax on the ability to drive or use machines is nil or negligible.
3. How to take Grazax
Follow exactly the administration instructions of this medicine indicated by your doctor. In case of doubt, consult your doctor or pharmacist again.
How much Grazax to take
- The recommended dose is one sublingual lyophilisate per day.
How to take Grazax
- To achieve greater efficacy, start taking the medicine 4 months before the expected start of the grass pollen season. It is recommended to continue treatment with Grazax for 3 years.
The first dose of Grazax should be taken in the doctor's office.
- This is because you should remain under observation for approximately half an hour after taking the first dose.
- This is a precaution to check your sensitivity to the medicine.
- This will give you the opportunity to discuss any side effects you may experience with your doctor.
Continue taking Grazax every day, even if it takes time for your allergy to improve.
If your allergy symptoms do not improve during the first grass pollen season, request a consultation with your doctor to discuss continuing treatment.
Make sure your hands are well dry before touching this medicine.
Take the sublingual lyophilisates as follows:
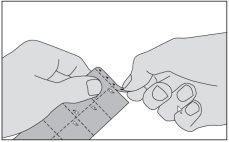
- Remove the strip marked with triangles at the top of the package.
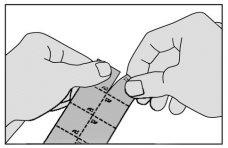
- Separate a unit from the package following the dotted lines.
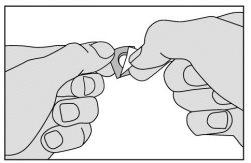
3. Fold the marked corner of the aluminum sheet and pull it.
Do not press to push this medicine through the aluminum sheet - it breaks easily.
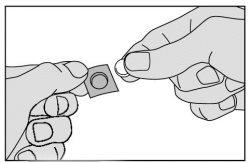
- Carefully remove this medicine from the unit and place it immediately under your tongue.
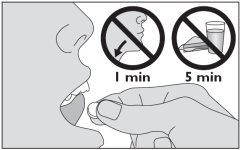
- Keep this medicine under your tongue until it dissolves.
- Do not swallow for one minute.
- Wait at least 5 minutes before eating or drinking after taking this medicine.
If you take more Grazax than you should
If you have taken too many Grazax sublingual lyophilisates, you may experience allergic symptoms, including local symptoms in the mouth and throat. If you experience severe symptoms, contact your doctor or hospital immediately.
If you forget to take Grazax
In case you forget to take a sublingual lyophilisate, take another as soon as possible on the same day. Do not take a double dose on the same day to make up for forgotten doses.
If you stop taking Grazax
If you do not take this medicine as prescribed by your doctor, it may not work effectively. If you have any other questions about the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Side effects can be an allergic-type reaction to the allergen being treated. In most cases, side effects last from minutes to hours after taking the sublingual lyophilisate and resolve within a week of starting treatment.
Serious side effects:
Stop taking Grazax and immediately go to your doctor or hospital if you experience any of the following side effects:
- Rapid swelling of the face, mouth, or throat
- Difficulty swallowing
- Difficulty breathing
- Hives
- Changes in voice
- Worsening of existing asthma
- Severe discomfort
If you suffer from persistent heartburn, you should contact your doctor.
Other possible side effects:
Very common (may affect more than 1 in 10 people):
- Mouth swelling
- Itching in the mouth or ear
- Irritation sensation in the throat
Common (may affect up to 1 in 10 people):
- Tingling or numbness sensation in the mouth
- Itching in the eyes, lips, or nose
- Inflammation in the eyes or mouth
- Difficulty breathing, coughing, or sneezing
- Throat dryness
- Nasal discharge
- Swelling of the eyes or lips
- Mouth ulcers
- Pain from blisters or discomfort in the mouth or throat
- Stomach pain, diarrhea, nausea, vomiting
- Heartburn
- Itching, rash, or hives
- Fatigue
- Chest discomfort
- Sensation of pressure in the throat
- Redness in the mouth
- Difficulty swallowing
Uncommon (may affect up to 1 in 100 people):
- Sensation of rapid, strong, or irregular heartbeats
- Taste alteration
- Redness or irritation of the eyes
- Pain or discomfort in the ear
- Sensation of numbness in the throat, pain when swallowing
- Tonsil inflammation
- Severe allergic reaction
- Mouth dryness
- Blisters on the lips, lip inflammation, lip ulcers
- Inflammation of the salivary glands or hypersalivation
- Stomach inflammation, regurgitation
- Sensation of a foreign body in the throat
- Redness of the skin
- Facial swelling
- Tongue inflammation
- Allergic reaction
- Tingling sensation on the skin
- Stomach discomfort
- Throat swelling
- Watery eyes
- Hoarseness
- Redness in the throat
- Blisters in the mouth
Rare (may affect up to 1 in 1000 people):
- Constriction of the lower airways
- Ear swelling
Irritation of the eyes, redness of the throat, blisters in the mouth, ear pain, and ear swelling have been reported more frequently in children than in adults.
If you experience bothersome side effects, you should contact your doctor, who will decide if you need to take anti-allergic medicines, such as antihistamines.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if it is a possible side effect not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System of Medicines for Human Use www.notificaRAM.es.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Grazax
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date stated on the blister and on the carton after EXP. The expiration date is the last day of the month indicated.
This medicine does not require special storage conditions.
Medicines should not be disposed of via wastewater or household waste. Place the packages and medicines you no longer need in the SIGRE  collection point at the pharmacy. If in doubt, ask your pharmacist how to dispose of packages and medicines you no longer need. This will help protect the environment.
collection point at the pharmacy. If in doubt, ask your pharmacist how to dispose of packages and medicines you no longer need. This will help protect the environment.
6. Contents of the pack and further information
Composition of Grazax
The active substance is a standardized allergen extract of grass pollen from Phleum pratense(Timothy grass), standardized in SQ units. The activity per sublingual lyophilisate is expressed using the SQ-T* unit. The activity of one sublingual lyophilisate is 75,000 SQ-T. The content of the Phl p 5 allergen per sublingual lyophilisate is 6 micrograms.
- (Sublingual lyophilisate in Standardized Quality (SQ-T) units.
The other ingredients are gelatin (from fish), mannitol, and sodium hydroxide.
Appearance of Grazax and contents of the pack
Round sublingual lyophilisate, white or off-white in color, with an engraving on one side.
The sublingual lyophilisates are packaged in an aluminum-aluminum blister with a foldable sheet for opening and packed in a cardboard box. Each blister contains 10 sublingual lyophilisates.
Available packages: 30 (3x10), 90 (9x10), or 100 (10x10) sublingual lyophilisates.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
ALK-Abelló A/S
Bøge Allé 6-8
DK-2970 Hørsholm
Denmark
Manufacturer
ALK-Abelló, S.A.
C/ Miguel Fleta, 19.
28037 – Madrid
Spain
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
ALK-Abelló, S.A.
C/ Miguel Fleta, 19.
28037 - Madrid
Date of the last revision of this leaflet:2024-12-19
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price106.84 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to GRAZAX 75000 SQ-T SUBLINGUAL LYOPHILIZEDDosage form: SUBLINGUAL TABLET, 300 IRActive substance: grass pollenManufacturer: StallergenesPrescription requiredDosage form: SUBLINGUAL TABLET, 100 IR/300 IRActive substance: grass pollenManufacturer: StallergenesPrescription requiredDosage form: ORALLY DISINTEGRATING TABLET/LIOTAB, 12 SQ-HDMActive substance: house dust mitesManufacturer: Alk-Abello A/SPrescription required
Online doctors for GRAZAX 75000 SQ-T SUBLINGUAL LYOPHILIZED
Discuss questions about GRAZAX 75000 SQ-T SUBLINGUAL LYOPHILIZED, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















