
FACTOR IX GRIFOLS 50 IU/ml POWDER AND SOLVENT FOR INJECTION

How to use FACTOR IX GRIFOLS 50 IU/ml POWDER AND SOLVENT FOR INJECTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Factor IX Grifols 50 UI/mlpowder and solvent for solution for injection
Human coagulation factor IX
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of thepackage leaflet
- What is Factor IX Grifols and what is it used for
- What you need to know before you use Factor IX Grifols
- How to use Factor IX Grifols
- Possible side effects
- Storage of Factor IX Grifols
- Contents of the pack and further information
1. What is Factor IX Grifols and what is it used for
Factor IX Grifols is a medicine that contains human coagulation factor IX.
Factor IX Grifols belongs to a group of medicines called antihemorrhagics: blood coagulation factors.
Factor IX Grifols is indicated for the treatment and prophylaxis (prevention) of bleeding in patients with hemophilia B (congenital factor IX deficiency). These patients do not have enough functional factor IX. Factor IX Grifols is used to increase the amount of factor IX in the blood, allowing it to clot.
2. What you need to know before you use Factor IX Grifols
Do not useFactor IX Grifols
If you are allergic to the active substance or to any of the other ingredients of this medicine (listed in section 6). See important information about some of the ingredients of Factor IX Grifols at the end of this section.
If you have any doubts about the above, consult your doctor.
Warnings and precautions
Consult your doctor, pharmacist, or nurse before starting to use Factor IX Grifols.
- There is a remote possibility that you may experience an anaphylactic reaction (severe allergic reaction). If you feel chest tightness, dizziness, vertigo, or nausea, or if you feel dizzy while standing, you may be suffering from an anaphylactic reaction to Factor IX Grifols. If this occurs, stop the administration of the product immediately and seek medical attention.
- If hypersensitivity reactions (allergy, e.g., fever, generalized urticaria, chest tightness, shortness of breath, hypotension, and anaphylaxis) occur during the administration of Factor IX Grifols, the injection/infusion must be interrupted. Your doctor will decide on the appropriate treatment (e.g., antihistamines, shock therapy).
- It is possible that your doctor may want to perform some tests to ensure that the dose of Factor IX Grifols you receive is sufficient to achieve and maintain adequate factor IX levels.
- If your bleeding is not controlled with Factor IX Grifols, consult your doctor immediately. You may have developed factor IX inhibitors, so your doctor will want to perform tests to confirm this. Factor IX inhibitors are antibodies present in the blood that block the factor IX you are using. This makes factor IX less effective in controlling bleeding.
- If you have a disease with a risk of thrombosis (history of heart disease or acute myocardial infarction, liver disease, thromboembolic disorders, coagulation disorders, or in newborn children) and if you are given high doses of factor IX in cases of major surgery. With adequate monitoring, possible complications can be detected in time and appropriate measures can be taken. Some of these complications are, for example, thromboembolism and consumption coagulopathy.
- If you need a central venous access device (CVAD) for the administration of Factor IX Grifols, your doctor should consider the risk of complications related to the CVAD, including local infections, presence of bacteria in the blood (bacteremia), and the formation of a blood clot in the blood vessel (thrombosis) where the catheter is inserted.
When medicines are prepared from human blood or plasma, a number of measures must be taken to prevent the possible transmission of infections to patients. These measures include:
- careful selection of blood and plasma donors to ensure the exclusion of donors at risk of infection,
- testing of each donation and plasma pools for possible viruses or infections,
- inclusion of a series of stages in the processing of blood or plasma that can inactivate or eliminate viruses.
Despite these measures, when medicines prepared from human blood or plasma are administered, the possibility of transmission of infections cannot be completely excluded. This also applies to unknown or emerging viruses and other types of infections.
The measures taken are considered effective for enveloped viruses such as human immunodeficiency virus (HIV), hepatitis B virus, and hepatitis C virus, and for the non-enveloped hepatitis A virus. The measures taken may have limited value for non-enveloped viruses such as parvovirus B19.
Parvovirus B19 infection can be severe for a pregnant woman (fetal infection) and for people whose immune system is depressed or who have some type of anemia (e.g., with sickle cell anemia or hemolytic anemia).
Your doctor may recommend that you consider vaccination against hepatitis A and B if you regularly receive human plasma-derived factor IX concentrates.
Each time you are administered a dose of Factor IX Grifols, it is recommended to keep a record of the name and batch number of the medicine to maintain a record of the batches used.
There may be a possible connection between the development of factor IX inhibitors and allergic reactions. Patients with factor IX inhibitors may have a higher risk of anaphylactic reactions. Therefore, in patients who suffer an allergic reaction, the presence of a factor IX inhibitor should be investigated.
Using Factor IX Grifols with other medicines
- Tell your doctor or pharmacist if you are using or have recently used or may need to use any other medicines.
- Currently, no interactions with other medicines are known.
- Factor IX Grifols should not be mixed with other medicines before administration, as it may adversely affect the efficacy and safety of the product.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
- Tell your doctor if you are pregnant or breastfeeding.
- Your doctor will decide if Factor IX Grifols can be used during pregnancy and breastfeeding.
- Since hemophilia B is rare in women, there is no experience with the use of Factor IX Grifols during pregnancy and breastfeeding.
Driving and using machines
There is no indication that Factor IX Grifols may affect the ability to drive or use machines.
Sodium content
Factor IX Grifols contains 20.7 mg of sodium in the 250 UI/5 ml presentation, 41.4 mg of sodium in the 500 UI/10 ml presentation, 82.8 mg of sodium in the 1000 UI/20 ml presentation, and 124.2 mg of sodium in the 1500 UI/30 ml presentation. This is equivalent to 1.04%, 2.07%, 4.14%, and 6.21%, respectively, of the maximum daily sodium intake recommended by the WHO for an adult (2 g of sodium).
3. How to use Factor IX Grifols
Reconstitute the product as described in this section. The product should be administered slowly, especially the first dose (approximately 3 ml/min) by intravenous route.
Follow exactly the administration instructions of this medicine indicated by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
Your doctor will decide the dose of Factor IX Grifols you will receive. This dose and its duration will depend on your individual needs for factor IX replacement therapy and pharmacokinetics (recovery and half-life), which should be regularly checked.
Your doctor may modify the dose of Factor IX Grifols you receive over time.
Dose for treatment
The required dose is determined using the following formula:
Required units = body weight (kg) x desired increase in factor IX (%) (UI/dl) x 0.8
Dose for prophylaxis in bleeding
In routine prophylaxis to prevent bleeding in patients with severe hemophilia B, doses of 20 to 40 UI of factor IX/kg of body weight should be administered at intervals of 3 to 4 days. In some cases, especially in young patients, it may be necessary to shorten the administration intervals or use higher doses.
Patients with inhibitors
If you have developed factor IX inhibitors, you may need a higher amount of Factor IX Grifols to control bleeding. If this dose does not control bleeding, your doctor may consider the use of an alternative medicine. Do not increase the total dose of Factor IX Grifols you use to control your bleeding without consulting your doctor.
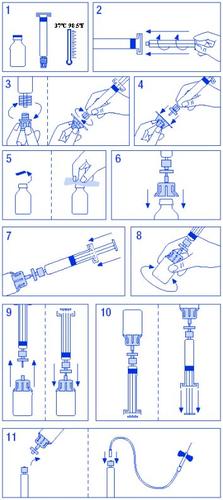
Instructions for use/handling
Follow these instructions unless your doctor has given you different instructions.
For the reconstitution and administration of Factor IX Grifols, 1500 UI/30 ml presentation, whose solvent is presented in vials, the preparation of the solution is as follows:
- Bring the vials to room temperature without exceeding 37 ºC.
- Open the vial of solvent, disinfecting the stopper with an alcohol wipe.
- Remove the double-pointed needle from the package. Separate one of the caps that protect the points and puncture the stopper of the vial of solvent.
- Open the vial of lyophilized product, disinfecting the stopper with an alcohol wipe.
- Separate the cap from the other point of the needle.
- Invert the vial of solvent and puncture the vial of lyophilized product, ensuring that all the solvent is transferred and avoiding loss of vacuum.
- Separate the solvent vial with the double-pointed needle. Gently turn the vial, avoiding the formation of foam, until the product is completely dissolved. Do not shake.
- Remove the filter from the blister pack and insert it into the syringe, loading the syringe with enough air for the total volume of the solution. Insert the needle into the filter and puncture the vial of the reconstituted product. Inject the pre-loaded air from the syringe, through the filter, and then invert the vial and aspirate the contents into the syringe.
- Remove the filter-needle assembly and administer slowly by intravenous route using the supplied butterfly needle at a speed of 3 ml/min.
For the reconstitution and administration of Factor IX Grifols, 250 UI/5 ml, 500 UI/10 ml, and 1000 UI/20 ml presentations, in which the solvent is presented in pre-filled syringes, the preparation of the solution is as follows:
- Bring the vial and syringe of solvent to room temperature without exceeding 37 ºC.
- Attach the plunger to the syringe of solvent.
- Open the filter. Separate the cap from the cone of the syringe of solvent and attach it to the filter.
- Open the vial adapter and attach it to the filter-syringe assembly.
- Open the vial, disinfecting the stopper with an alcohol wipe.
- Insert the spike of the adapter into the vial.
- Transfer all the solvent from the syringe to the vial.
- Gently turn the vial, avoiding the formation of foam, until the product is completely dissolved. Do not shake.
- Separate the filter-syringe assembly from the rest. Aspirate enough air for the total volume of the solution. Reattach the filter-syringe assembly to the vial.
- Invert the vial and aspirate the contents into the syringe.
- Separate the syringe and administer slowly by intravenous route using the supplied butterfly needle at a speed of 3 ml/min.
It is important to use the injection equipment supplied with the medicine. If medical infusion equipment is used, check the compatibility of the system with the pre-filled syringe. To ensure adequate administration of the product, an adapter may be required in some cases.
Reconstitution scheme for solvent in syringes
If you use moreFactor IX Grifolsthan you should
No cases of overdose with human coagulation factor IX have been reported. However, if you have used Factor IX Grifols more than you should, consult your doctor or pharmacist immediately.
In case of overdose or accidental administration, consult the Toxicological Information Service, phone 91 562 04 20.
If you forget to use Factor IX Grifols
- Do not take a double dose to make up for forgotten doses.
- Continue with the next administration immediately and follow a regular schedule as indicated by your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
In rare cases, you may notice one of the following side effects after administration of Factor IX Grifols:
- Itching, local reactions at the injection site (e.g., burning sensation and transient redness)
- Allergic reactions (e.g., chest tightness/general feeling of discomfort, dizziness, nausea, and slight drop in blood pressure that may cause dizziness while standing)
It cannot be completely excluded that an anaphylactic shock may occur. If you notice any of the following symptoms during injection/infusion
- chest tightness/general feeling of discomfort
- dizziness
- mild hypotension (slight decrease in blood pressure with dizziness while standing)
- nausea
it may be an early sign of hypersensitivity and anaphylactic reaction. If an allergic reaction or anaphylaxis occurs, the injection/infusion must be interrupted and your doctor consulted immediately.
However, it cannot be completely excluded that there is a possibility of allergic reactions to the components of the preparation. The formation of neutralizing antibodies against factor IX (inhibitors) is a known complication in the treatment of patients with hemophilia B. The development of inhibitors should be carefully monitored by laboratory tests and appropriate clinical examinations to determine the formation of such inhibitors.
There is a risk of thromboembolic complications with Factor IX Grifols, particularly if you have a risk of thrombosis and/or receive high-dose therapy.
- For information on viral safety, see section 2.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Medicines Vigilance System for Human Use: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Factor IX Grifols
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the carton after the abbreviation “EXP”. The expiry date refers to the last day of the month stated.
Vial of lyophilized powder (human coagulation factor IX): store between 2 ºC and 8 ºC (in a refrigerator).
Vial or syringe of solvent (water for injections): store between 2 ºC and 30 ºC.
When outpatient administration is required, the product can be stored at room temperature (not above 25 ºC) for a single period of 3 months maximum.
The product should not be refrigerated again after being stored at room temperature.
Do not use this medicine if you observe that the solution is turbid or contains sediment. The solution is usually clear or slightly opalescent.
Once reconstituted, the solution should be discarded if particles are observed inside or if any discoloration occurs.
The reconstituted solution should be used immediately or within 3 hours.
Any unused product and waste material should be disposed of in accordance with local requirements.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Composition ofFactor IX Grifols
The active ingredient is human coagulation factor IX.
Each vial of Factor IX Grifols contains lyophilized powder with 250 IU, 500 IU, 1000 IU, or 1500 IU of human coagulation factor IX. Once reconstituted, the human factor IX content is 50 IU/ml (250 IU/5 ml, 500 IU/10 ml, 1000 IU/20 ml, or 1500 IU/30 ml).
The other components are lysine, glycine, chloride, sodium, phosphate, and citrate.
Each solvent container contains 5 ml, 10 ml, 20 ml, or 30 ml of water for injections.
See section 2 for important information about some of the components.
Appearance of the Product and Container Contents
Vial containing white or pale yellow powder and vial/syringe with water for injections (solvent).
Each vial of Factor IX Grifols in the 250 IU/5 ml, 500 IU/10 ml, and 1000 IU/20 ml presentations comes with a pre-loaded syringe of solvent containing 5 ml, 10 ml, or 20 ml of water for injections, along with the necessary accessories for reconstitution and administration (vial adapter, filter, 2 alcohol swabs, and butterfly needle).
Each vial of Factor IX Grifols in the 1500 IU/30 ml presentation comes with a vial of solvent with 30 ml of water for injections, along with the necessary accessories for reconstitution and administration (double-pointed needle, filter, 2 alcohol swabs, butterfly needle, and syringe with needle).
Only some package sizes may be marketed.
Box contents: 1 lyophilized vial, 1 pre-loaded syringe/vial of solvent, and accessories.
Marketing Authorization Holder and Manufacturer
Instituto Grifols, S.A.
Can Guasch, 2 - Parets del Vallès
08150 Barcelona - SPAIN
Date of the Last Revision of this Prospectus: April 2019
Detailed information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/).
……………………………………………………………………………………………………………
This information is intended only for healthcare professionals:
The following table can be used as a dosing guide in hemorrhagic episodes and surgery.
Severity of Hemorrhage/ Type of Surgery | Required Factor IX Level (%)(IU/dl) | Dosing Frequency (hours)/Duration of Therapy (days) |
Hemorrhage | ||
Mild hemarthrosis and muscle or oral bleeding | 20 - 40 | Repeat every 24 hours. At least 1 day, until the hemorrhagic episode manifested by pain stops or until healing. |
Moderate hemarthrosis and muscle or hematoma bleeding | 30 - 60 | Repeat infusion every 24 hours for 3 - 4 days or more until pain and acute disability disappear. |
Life-threatening hemorrhages | 60 - 100 | Repeat infusion every 8 - 24 hours until the risk disappears. |
Surgery | ||
Minor surgery including dental extractions Major surgery | 30 - 60 80 - 100 (pre- and postoperative) | Every 24 hours, at least 1 day until healing. Repeat infusion every 8 - 24 hours until adequate wound healing, and then treatment for at least 7 days to maintain a factor IX activity level of 30% to 60% (IU/dl). |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FACTOR IX GRIFOLS 50 IU/ml POWDER AND SOLVENT FOR INJECTIONDosage form: INJECTABLE, 1,000 IUActive substance: coagulation factor IXManufacturer: Swedish Orphan Biovitrum Ab (Publ)Prescription requiredDosage form: INJECTABLE, 2,000 IUActive substance: coagulation factor IXManufacturer: Swedish Orphan Biovitrum Ab (Publ)Prescription requiredDosage form: INJECTABLE, 250 IUActive substance: coagulation factor IXManufacturer: Swedish Orphan Biovitrum Ab (Publ)Prescription required
Online doctors for FACTOR IX GRIFOLS 50 IU/ml POWDER AND SOLVENT FOR INJECTION
Discuss questions about FACTOR IX GRIFOLS 50 IU/ml POWDER AND SOLVENT FOR INJECTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















