ENGERIX-B 20 micrograms/1 ml, PRE-FILLED SYRINGE SUSPENSION FOR INJECTION

How to use ENGERIX-B 20 micrograms/1 ml, PRE-FILLED SYRINGE SUSPENSION FOR INJECTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Engerix-B 20 micrograms/1 ml suspension for injection in pre-filled syringe
Hepatitis B vaccine (rDNA, adsorbed) (HBV)
Read all of this leaflet carefully before you start receiving this vaccine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This vaccine has been prescribed for you only. Do not pass it on to others.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See Section 4.
Contents of the pack
- What is Engerix-B and what is it used for
- What you need to know before you receive Engerix-B
- How Engerix-B is administered
- Possible side effects
- Storage of Engerix-B
- Contents of the pack and other information
1. What is Engerix-B and what is it used for
Engerix-B is a vaccine that is used to protect against hepatitis B infection. It may also help protect against hepatitis D infection.
This vaccine can be given to adults and adolescents from 16 years of age. In exceptional circumstances, it may also be given to children and adolescents between 11 and 15 years of age (see section 3).
Hepatitis B is an infectious disease of the liver caused by a virus. Some people have the hepatitis B virus in their body but cannot get rid of it. These people can continue to infect others and are known as carriers. The spread of the disease occurs when the virus enters the body after contact with bodily fluids, almost always blood, from an infected person.
If a mother is a carrier of the virus, she can pass the virus to her child during birth. It is also possible to contract the virus from a carrier through, for example, unprotected sex, sharing injectable syringes, or treatment with medical equipment that has not been properly sterilized.
The main signs of the disease include: headache, fever, malaise (nausea) and jaundice (the skin and eyes turn yellow), although in about three out of 10 patients there are no signs of disease. Of those infected with hepatitis B, one in 10 adults and up to nine in 10 children will become carriers of the virus and are likely to develop severe liver damage and in some cases liver cancer.
How Engerix-B works
Engerix-B contains a small amount of the outer surface of the hepatitis B virus. This outer surface is not infectious and cannot cause the disease.
- When you receive the vaccine, it will stimulate your body's immune system to prepare it to protect itself against these viruses in the future.
- Engerix-B will not protect you if you have already contracted the hepatitis B virus.
- Engerix-B can only help protect you against hepatitis B virus infection.
2. What you need to know before you receive Engerix-B
Do not receive Engerix-B:
- If you are allergic (hypersensitive) to Engerix-B or any of the other components of this vaccine (listed in section 6).
- If you have a high temperature (fever).
Engerix-B should not be administered if any of the above applies to you. If you are not sure, talk to your doctor or pharmacist before receiving Engerix-B. Inform your doctor or pharmacist if you have any allergies or if you have ever had health problems after receiving a vaccine.
Warnings and precautions
Talk to your doctor or pharmacist before receiving Engerix-B if:
- You are on dialysis for a kidney problem or if you have a disease that may affect your immune system.
People who need dialysis, who have chronic liver problems, who are hepatitis C carriers, or who are HIV positive can also receive Engerix-B. This is because hepatitis B infections can be severe in these patients. You will find more information about kidney problems and dialysis in Section 3.
If you are not sure if any of the above applies to you, talk to your doctor before receiving Engerix-B.
Fainting (syncope) may occur after any injection, especially in adolescents. You should inform your doctor or nurse if you or your child have fainted after a previous injection.
Like other vaccines, Engerix-B may not fully protect you against hepatitis B. A number of factors such as advanced age, sex, overweight, smoking, and some chronic problems reduce the immune response to the vaccine. If any of these apply to you, your doctor may decide to perform a blood test or administer additional doses of Engerix-B to ensure you are protected.
Other medicines and Engerix-B
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Engerix-B can be administered at the same time as most usual vaccines. Your doctor will make sure that the vaccines are injected separately and in different parts of the body.
Pregnancy and breastfeeding
- If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Driving and using machines
It is unlikely that Engerix-B will affect your ability to drive or use machines. However, do not drive or use machines if you feel unwell.
Engerix-B contains sodium
This vaccine contains less than 23 mg of sodium (1 mmol) per dose; this is essentially "sodium-free".
3. How Engerix-B is administered
How your vaccine is administered
Your doctor will administer the recommended dose of Engerix-B.
Engerix-B will be administered:
- as an injection into the muscle of the upper arm
- as an injection under the skin if you bruise easily or have bleeding problems.
How much is administered
You will receive a series of Engerix-B injections. Once you have completed the injection schedule, you can expect long-term protection against hepatitis B.
Adults and adolescents from 16 years of age will receive the 20 micrograms/1 ml vaccine.
There are several alternatives for administering Engerix-B. Your doctor will choose the most suitable schedule for you:
Schedule 1 – for adults or adolescents from 16 years of age
First injection - now
Second injection - 1 month after the first injection
Third injection - 6 months after the first injection
Schedule 2 – for adults or adolescents from 16 years of age
First injection - now
Second injection - 1 month after the first injection
Third injection - 2 months after the first injection
Fourth injection - 12 months after the first injection
- This schedule can also be used if you are being vaccinated due to recent exposure to hepatitis B, as it will provide faster protection.
Schedule 3 – only for adults (from 18 years of age)
This schedule will only be administered in exceptional circumstances, for example, if you need to travel to a high-risk area within one month of being vaccinated.
First injection - now
Second injection - 1 week after the first injection
Third injection - 3 weeks after the first injection
Fourth injection - 12 months after the first injection
Schedule 4 – only for children and adolescents between 11 and 15 years of age
This schedule is only used if there is doubt that the child will receive the third injection. For this schedule, Engerix-B (20 micrograms/1 ml) will be used. This will provide a higher level of protection than two doses of Engerix-B Junior (10 micrograms/0.5 ml).
First injection - now
Second injection - 6 months after the first injection
- When using this schedule, protection may not be achieved until after the second dose. This two-dose schedule is only used when there is a relatively low risk of hepatitis B infection during the vaccination schedule and when completion of the schedule can be ensured.
It is very important that you attend your injections at the recommended intervals. If you have any questions about the amount of vaccine you will receive, consult your doctor.
Kidney problems and dialysis
- People from 16 years of age
If you have a kidney problem or are on dialysis, your doctor may decide to vaccinate you with four double doses (2 x 20 micrograms/1 ml) of vaccine at 0, 1, 2, and 6 months. Your doctor may also decide to perform a blood test to ensure you are protected against hepatitis B.
4. Possible side effects
Like all vaccines, this vaccine can cause side effects, although not everybody gets them. The following side effects may occur with this vaccine:
Allergic reactions(these may occur in up to 1 in 10,000 doses of the vaccine)
If you have an allergic reaction, see a doctor immediately. The signs may include:
- swelling of the face
- low blood pressure
- difficulty breathing
- the skin turns blue
- loss of consciousness.
These signs usually start very soon after the injection is given. See a doctor immediately if they occur after you leave the clinic.
Other side effects include:
Very common(these may occur in more than 1 in 10 doses of the vaccine): pain and redness at the injection site, feeling tired, irritability.
Common(these may occur in up to 1 in 10 doses of the vaccine): headache, numbness, nausea or vomiting, diarrhea or abdominal pain, loss of appetite, fever (high body temperature), feeling unwell, swelling at the injection site, reactions at the injection site, such as induration.
Uncommon(these may occur in up to 1 in 100 doses of the vaccine): dizziness, muscle pain, flu-like symptoms.
Rare(these may occur in up to 1 in 1,000 doses of the vaccine): swollen glands, hives, skin rash and itching, joint pain, tingling.
Very rare(these may occur in up to 1 in 10,000 doses of the vaccine): easy bruising and inability to stop bleeding if you cut yourself, low blood pressure, inflammation of blood vessels, sudden swelling of the face around the mouth and throat (angioneurotic edema), inability to move muscles (paralysis), inflammation of nerves (neuritis) that can cause loss of sensation or numbness, including temporary inflammation of nerves, causing pain, weakness, and paralysis in the limbs and often progressing to the chest and face (Guillain-Barré syndrome), a disease of the nerves of the eye (optic neuritis) and multiple sclerosis, problems moving arms or legs (neuropathy), inflammation of the brain (encephalitis), degenerative brain disease (encephalopathy), infection around the brain (meningitis), seizures (convulsions), loss of skin sensation to pain or touch (hypoesthesia), purple or purple-red spots on the skin (lichen planus), red or purple spots on the skin, pain and stiffness in the joints (arthritis), muscle weakness.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Medicines Agency's website, www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Engerix-B
- Keep this vaccine out of the sight and reach of children.
- Do not use this vaccine after the expiry date which is stated on the label and carton, after EXP. The expiry date is the last day of the month shown.
- Store in a refrigerator (between 2°C and 8°C).
- Do not freeze.
- Store in the original package to protect from light.
- Medicines should not be disposed of via wastewater or household waste. Dispose of the container and any unused medicine in the SIGRE collection point at your pharmacy. If you are unsure, ask your pharmacist how to dispose of the container and any unused medicine. This will help protect the environment.
6. Contents of the pack and other information
Composition of Engerix-B
- The active substance is the outer surface of the hepatitis B virus. Each dose contains 20 micrograms/1 ml of protein composed of this outer surface, adsorbed onto hydrated aluminum hydroxide.
- The other ingredients are sodium chloride, disodium phosphate dihydrate, dibasic sodium phosphate, and water for injections.
Appearance and pack contents
Engerix-B is a white, turbid liquid.
Engerix-B (20 micrograms/ml) is available in a pre-filled syringe with or without separate needles; pack sizes of 1 and 10.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
GlaxoSmithKline, S.A.
P.T.M. C/ Severo Ochoa, 2
28760 Tres Cantos (Madrid)
Tel.: +34 900 202 700
Manufacturer
GlaxoSmithKline Biologicals S.A.
Rue de l’Institut 89
B-1330 Rixensart
Belgium
or
SmithKline Beecham, S.A.
Carretera de Ajalvir. Km. 2,5
28806 Alcalá de Henares
Madrid
This medicine is authorized in the Member States of the European Economic Area under the following names:
Austria, Denmark, Spain, Finland, Netherlands, Iceland, Norway, Sweden: Engerix-B
Belgium, Luxembourg, Portugal: Engerix B
France, Ireland, Italy: Engerix B-20
Germany: Engerix-B Adult
Greece: Engerix
Date of last revision of this leaflet:02/2024
Other sources of information
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/).
---------------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
Once stored, the content may appear as a fine white deposit with a clear colorless supernatant. After shaking, the vaccine is slightly opaque.
Before using the vaccine, it should be visually inspected for foreign particles and/or abnormal physical appearance. Do not administer the vaccine if you observe either of these.
All contents of the monodose container should be withdrawn and used immediately after withdrawal.
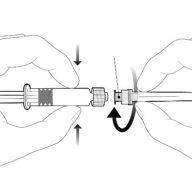
Instructions for the pre-filled syringe
| Hold the syringe by the body, not by the plunger. Remove the syringe cap by twisting it in an anti-clockwise direction. |
| To insert the needle, attach the base to the luer-lock adapter and twist it a quarter turn in a clockwise direction until you feel it click. Do not pull the plunger out of the syringe body. If this happens, do not administer the vaccine. |
Disposal of waste
Disposal of unused medicine and all materials that have come into contact with it will be carried out in accordance with local regulations.
- Country of registration
- Average pharmacy price16.77 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ENGERIX-B 20 micrograms/1 ml, PRE-FILLED SYRINGE SUSPENSION FOR INJECTIONDosage form: INJECTABLE, 10 mcg Hepatitis B Surface Antigen / 0.5 mlActive substance: hepatitis B, purified antigenManufacturer: Glaxosmithkline S.A.Prescription requiredDosage form: INJECTABLE, 20 µgActive substance: hepatitis B, purified antigenManufacturer: Glaxosmithkline BiologicalsPrescription requiredDosage form: INJECTABLE, 20 µgActive substance: hepatitis B, purified antigenManufacturer: Glaxosmithkline BiologicalsPrescription required
Online doctors for ENGERIX-B 20 micrograms/1 ml, PRE-FILLED SYRINGE SUSPENSION FOR INJECTION
Discuss questions about ENGERIX-B 20 micrograms/1 ml, PRE-FILLED SYRINGE SUSPENSION FOR INJECTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions