EMGALITY 120 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE

How to use EMGALITY 120 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Emgality 120mg solution for injection in pre-filled syringe
galcanezumab
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Emgality and what is it used for
- What you need to know before you use Emgality
- How to use Emgality
- Possible side effects
- Storage of Emgality
- Contents of the pack and other information
1. What is Emgality and what is it used for
Emgality contains galcanezumab, a medicine that blocks the activity of a naturally occurring substance in the body called calcitonin gene-related peptide (CGRP). People with migraine may have increased levels of CGRP.
Emgality is used to prevent migraine in adults who have at least 4 days of migraine per month.
Emgality may reduce the frequency of migraine and improve your quality of life. It starts to work in about one week.
2. What you need to know before you use Emgality
Do not use Emgality:
- if you are allergic to galcanezumab or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before or during treatment with Emgality if:
- you have severe cardiovascular disease. Emgality has not been studied in patients with severe cardiovascular diseases.
Monitor for signs of allergic reactions
Emgality may cause potentially serious allergic reactions. Serious allergic reactions usually occur within 1 day after using Emgality, but some reactions may be delayed (occurring more than 1 day and up to 4 weeks after using Emgality). Some allergic reactions can be long-lasting. You should monitor for signs of these reactions while using Emgality. Stop using Emgality and tell your doctor or seek medical attention immediately if you notice any signs of a serious allergic reaction. These signs are listed under “Serious side effects” in section 4.
Children and adolescents
This medicine must not be given to children and adolescents under 18 years because it has not been studied in this age group.
Other medicines and Emgality
Tell your doctor, pharmacist, or nurse if you are using, have recently used, or might use any other medicines.
Pregnancy and breastfeeding
If you are a woman who could become pregnant, you are advised to avoid becoming pregnant while using Emgality.
If you are pregnant or think you may be pregnant, talk to your doctor before using this medicine. It is preferable to avoid using Emgality during pregnancy, as the effects of this medicine in pregnant women are not known.
If you are breastfeeding or plan to breastfeed, talk to your doctor before using this medicine. You and your doctor must decide whether to breastfeed or use Emgality.
Driving and using machines
Galcanezumab may have a small effect on your ability to drive and use machines. Some patients have experienced dizziness while using Emgality.
Emgality contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per 120 mg dose; it is essentially “sodium-free”.
3. How to use Emgality
Follow exactly the administration instructions for this medicine given by your doctor, pharmacist, or nurse. If you are unsure, talk to your doctor, pharmacist, or nurse again.
The Emgality pre-filled syringe is for single use and contains one dose of Emgality (120 mg).
- The first time you are given Emgality, your doctor or nurse will inject two syringes (240 mg in total).
- After the first dose, you will use one syringe (120 mg) every month.
Your doctor will decide how long you should use Emgality.
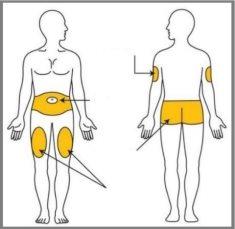
Emgality is given by injection under the skin (subcutaneous injection). You and your doctor or nurse must decide if you can inject Emgality yourself.
It is important that you do not try to inject yourself until you have been trained on how to do it by your doctor or nurse. A caregiver can also give you your Emgality injection if they are properly trained.
The syringe must not be shaken.
Read the “Instructions for Use” of the syringe carefully before using Emgality.
If you use more Emgality than you should
If you have injected more Emgality than you should, e.g., if after the first dose of 240 mg you have injected the medicine twice in one month, or if someone else has used Emgality accidentally, talk to your doctor immediately.
If you forget to use Emgality
Do not inject a double dose to make up for a forgotten injection.
If you have forgotten to inject a dose of Emgality, inject the missed dose as soon as possible and then inject the next dose after one month from that date.
If you stop using Emgality
Do not stop using Emgality without talking to your doctor first.
If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious side effects
Generally, allergic reactions with Emgality are mild to moderate (such as rash or itching). Serious allergic reactions can occur rarely (may affect up to 1 in 1000 people) and signs may include:
- difficulty breathing or swallowing,
- low blood pressure, which can cause dizziness or fainting,
- swelling of the neck, face, mouth, lips, tongue, or throat that can develop rapidly,
- intense itching of the skin with rash or hives.
Talk to your doctor or seek medical attention immediately if you notice any of these signs.
Other side effects reported
Very common side effects(may affect more than 1 in 10 people):
- Pain at the injection site
- Injection site reactions (e.g., red skin, itching, bruising, swelling)
Common side effects(may affect up to 1 in 10 people):
- Dizziness (a feeling of spinning or dizziness)
- Constipation
- Itching
- Rash
Uncommon side effects(may affect up to 1 in 100 people):
- Hives (itchy rash on the skin)
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Emgality
Keep this medicine out of the sight and reach of children.
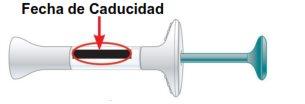
Do not use this medicine after the expiry date which is stated on the label and carton after EXP. The expiry date is the last day of the month shown.
Store in a refrigerator (between 2°C and 8°C). Do not freeze.
Keep the pre-filled syringe in the original package to protect it from light.
Emgality can be left outside the refrigerator for a single period of up to 7 days at a temperature not above 30°C. The syringe should be discarded if stored at a higher temperature or for a longer period.
Do not use this medicine if you notice that the syringe is damaged or the medicine appears cloudy or has particles in it.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and additional information
Composition of Emgality
- The active substance is galcanezumab. Each pre-filled syringe contains 120 mg of galcanezumab in 1 ml of solution.
- The other ingredients are: L-histidine, L-histidine hydrochloride monohydrate, polysorbate 80, sodium chloride, and water for injectable preparations.
Appearance and pack contents of the product
Emgality is an injectable solution in a single-dose, transparent glass syringe. Its color may vary from colorless to slightly yellow. Pack sizes of 1, 2, or 3 pre-filled syringes.
Only some pack sizes may be marketed.
Marketing authorisation holder
Eli Lilly Nederland B.V., Papendorpseweg 83, 3528 BJ Utrecht, Netherlands.
Manufacturer
Eli Lilly Italia S.p.A., Via Gramsci 731/733, 50019, Sesto Fiorentino (FI), Italy.
You can request more information about this medicine by contacting the local representative of the marketing authorisation holder:
Belgique/België/Belgien Eli Lilly Benelux S.A./N.V. Tél/Tel: + 32-(0)2 548 84 84 | Lietuva Eli Lilly Lietuva Tel. +370 (5) 2649600 |
| Luxembourg/Luxemburg Eli Lilly Benelux S.A./N.V. Tél/Tel: + 32-(0)2 548 84 84 |
Ceská republika ELI LILLY CR, s.r.o. Tel: + 420 234 664 111 | Magyarország Lilly Hungária Kft. Tel: + 36 1 328 5100 |
Danmark Eli Lilly Danmark A/S Tlf: +45 45 26 60 00 | Malta Charles de Giorgio Ltd. Tel: + 356 25600 500 |
Deutschland Lilly Deutschland GmbH Tel. + 49-(0) 6172 273 2222 | Nederland Eli Lilly Nederland B.V. Tel: + 31-(0) 30 60 25 800 |
Eesti Eli Lilly Nederland B. V. Tel: +372 6 817 280 | Norge Eli Lilly Norge A.S. Tlf: + 47 22 88 18 00 |
Ελλάδα ΦΑΡΜΑΣΕΡΒ-ΛΙΛΛΥ Α.Ε.Β.Ε. Τηλ: +30 210 629 4600 | Österreich Eli Lilly Ges.m.b.H. Tel: + 43-(0) 1 711 780 |
España Organon Salud, S.L. Tel: +34 91 591 12 79 | Polska Eli Lilly Polska Sp. z o.o. Tel: +48 22 440 33 00 |
France Organon France Tél: +33-(0) 1 57 77 32 00 | Portugal Lilly Portugal Produtos Farmacêuticos, Lda Tel: + 351-21-4126600 |
Hrvatska Eli Lilly Hrvatska d.o.o. Tel: +385 1 2350 999 | România Eli Lilly România S.R.L. Tel: + 40 21 4023000 |
Ireland Eli Lilly and Company (Ireland) Limited Tel: + 353-(0) 1 661 4377 | Slovenija Eli Lilly farmacevtska družba, d.o.o. Tel: +386 (0)1 580 00 10 |
Ísland Icepharma hf. Sími + 354 540 8000 | Slovenská republika Eli Lilly Slovakia s.r.o. Tel: + 421 220 663 111 |
Italia Eli Lilly Italia S.p.A. Tel: + 39- 055 42571 | Suomi/Finland Oy Eli Lilly Finland Ab Puh/Tel: + 358-(0) 9 85 45 250 |
Κύπρος Phadisco Ltd Τηλ: +357 22 715000 | Sverige Eli Lilly Sweden AB Tel: + 46-(0) 8 7378800 |
Latvija Eli Lilly (Suisse) S.A Parstavnieciba Latvija Tel: +371 67364000 | United Kingdom(Northern Ireland) Eli Lilly and Company (Ireland) Limited Tel: + 353-(0) 1 661 4377 |
Date of last revision of this leaflet:
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency web site: http://www.ema.europa.eu/.
Instructions for use |
Emgality 120mg solution for injection in pre-filled syringe |
galcanezumab |
For subcutaneous use |
|
Before using your pre-filled syringe: |
Importantinformation |
|
|
|
|
|
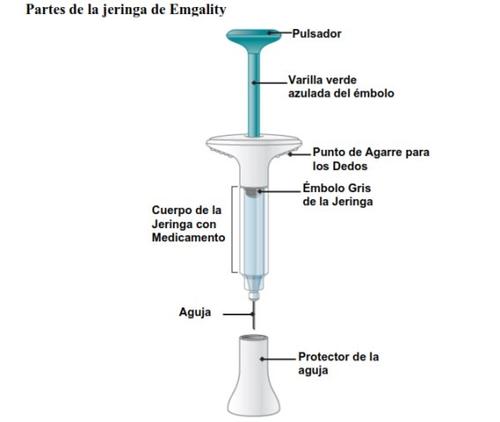
INSTRUCTIONS FOR USE
Before using the Emgality syringe, read and follow all the step-by-step instructions carefully.
Before you start | |
Take the syringe out of the refrigerator | Put the original package with the remaining unused syringes back in the refrigerator. |
Leave the needle protector on the syringe until you are ready to inject. | |
Do notshake. For a more comfortable injection, leave the syringe at room temperature for 30 minutes before injecting. Do notput the syringe in the microwave, run hot water over it, or put it in direct sunlight. | |
Inspect the syringe and medicine | Make sure you have the correct medicine. The medicine inside should be clear. The color may vary from colorless to slightly yellow. |
Do notuse the syringe and dispose of it as instructed by your doctor, pharmacist, or nurse if:
| |
|
Prepare to inject | Wash your hands with water and soap before injecting Emgality. Make sure you have a sharps container nearby. |
Choose your injection site | Your doctor, pharmacist, or nurse can help you choose the best injection site for you. |
|
|
| |
| |
|
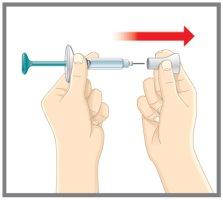
1 | Remove the cap |
|
| ||
| ||
| ||
| ||
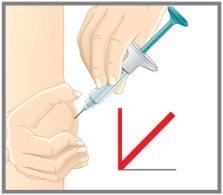
2 | Insert |
|
| ||
|
3 | Inject |
|
| ||
| ||
|
| Blue-green plunger rod |
Syringe gray plunger |
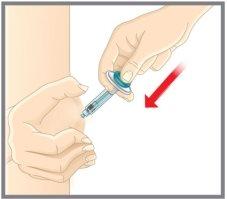
After injecting your medicine | |
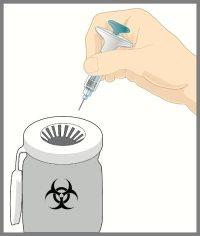
Dispose of the syringe | |
DO NOT put the needle protector back on the syringe. Dispose of the syringe in a sharps container or as instructed by your doctor, pharmacist, or nurse. |
|
When disposing of the syringe and sharps container:
| |
Frequently asked questions | |
Q. | What if I see air bubbles in my Emgality syringe? |
A. | It is normal to have air bubbles in the syringe. Emgality is injected under the skin (subcutaneous injection). |
Q. | What if there is a drop of liquid on the tip of the needle when I remove the needle protector? |
A. | A drop of liquid on the tip of the needle is not unusual. |
Q. | What do I do if I cannot push the plunger? |
A. | If the plunger is stuck or damaged:
|
Q. | What if there is a drop of liquid or blood on my skin after injection? |
A. | This is normal. Press a cotton ball or gauze over the injection site. Do not rub the injection site. |
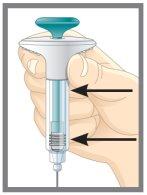
Q. | How do I know when the injection is complete? |
A. | Your injection is complete when: |
| |
|
To learn more about your medicine, read the complete Emgality leaflet inside this package.
Revision date:
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to EMGALITY 120 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGEDosage form: INJECTABLE, 120 mg/mlActive substance: galcanezumabManufacturer: Eli Lilly Nederland B.V.Prescription requiredDosage form: INJECTABLE, 140 mgActive substance: erenumabManufacturer: Novartis Europharm LimitedPrescription requiredDosage form: INJECTABLE, 70 mgActive substance: erenumabManufacturer: Novartis Europharm LimitedPrescription required
Online doctors for EMGALITY 120 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE
Discuss questions about EMGALITY 120 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions