DYNAMOGÉN 3 MG/1 G/10ML ORAL SOLUTION


How to use DYNAMOGÉN 3 MG/1 G/10ML ORAL SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
DYNAMOGÉN 3 mg/1 g/10ml Oral Solution
?-ketoglutarate of cyproheptadine/arginine aspartate
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Dynamogén and what is it used for
- What you need to know before you take Dynamogén
- How to take Dynamogén
- Possible side effects
- Storage of Dynamogén
- Contents of the pack and further information
1. What is Dynamogén and what is it used for
Dynamogén belongs to a group of medicines called appetite stimulants.
Dynamogén is indicated for the symptomatic treatment of loss of appetite in adults and children over 2 years of age.
2. What you need to know before you take Dynamogén
Do not take Dynamogén
- if you are allergic to cyproheptadine, arginine aspartate or any of the other ingredients of this medicine (listed in section 6).
- in children under 2 years of age.
- if you have severe kidney or liver disease.
- in cases of asthma attack.
- if you are pregnant or breastfeeding.
- if you have glaucoma (increased pressure inside the eye), enlarged prostate, problems with emptying the gallbladder or stomach, or difficulty urinating.
- if you are taking a type of medicine called "monoamine oxidase inhibitor" (MAOI) for depression or Parkinson's disease.
- if you have porphyria (a rare, usually inherited disorder in which large amounts of porphyrin are excreted in urine and feces).
Warnings and precautions
Consult your doctor or pharmacist before starting to take Dynamogén.
Do not take this medicine continuously for more than 8 weeks.
If your symptoms worsen or persist after 4 weeks, stop taking the medicine and consult your doctor.
- If you experience bruising, bleeding, pallor, fever, or sore throat during treatment, consult your doctor.
- Consult your doctor if you are taking antibiotics, as antihistamines like cyproheptadine may mask the first signs of ototoxicity (hearing loss) that some antibiotics have.
- Consult your doctor before taking this medicine if you have high blood pressure, an overactive thyroid gland, high blood pressure, heart problems, or asthma.
- Special attention should be given when determining the dose of Dynamogén in children over 2 years of age due to the greater sensitivity to antihistamines in this population.
- Some antihistamines may decrease attention and, in the case of children, may occasionally cause excitement.
- Overdose of some antihistamines in children can cause alterations of the nervous system, respiratory arrest, and cardiac arrest, and can even be fatal (see the section "If you take more Dynamogén than you should").
Other medicines and Dynamogén
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines, including those obtained without a prescription.
Consult your doctor if you are taking a type of medicine called "monoamine oxidase inhibitors" (MAOIs), oral contraceptives, or central nervous system depressants such as alcohol, benzodiazepines, or barbiturates.
Dynamogén may reduce the effect of some antidepressants.
Interference with diagnostic tests: false negatives may appear in allergy tests and an increase in triglycerides in blood. If Dynamogén is administered with antibiotics, false negative results may appear in vitamin B12 values and in the number of red blood cells.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Do not use Dynamogén during pregnancy or breastfeeding.
Driving and using machines
Dynamogén may cause drowsiness and therefore decrease concentration and reflexes, although this effect usually disappears after a few days of continuous administration of the medicine. Therefore, driving vehicles or operating hazardous or precision machinery is not recommended when taking this medicine.
Dynamogén contains sucrose, sorbitol (E-420), and amaranth dye (E-123)
This medicine contains sucrose and sorbitol (E-420). If your doctor has told you that you have an intolerance to certain sugars, consult with them before taking this medicine.
Patients with diabetes mellitus should note that this medicine contains 3.575 g of sucrose per 10 ml ampoule.
It may produce a slight laxative effect because it contains 3.41 g of sorbitol per 10 ml ampoule.
Caloric value: 2.6 kcal/g of sorbitol.
This medicine may cause allergic reactions because it contains amaranth dye (E-123). It can cause asthma, especially in patients allergic to acetylsalicylic acid.
3. How to take Dynamogén
Follow exactly the administration instructions of this medicine indicated by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
This medicine is administered orally.
The recommended dose is:
Adults and adolescents over 14 years: 1 drinkable ampoule 3 times a day. Each ampoule contains 2 mg of cyproheptadine, which is equivalent to 6 mg per day. Do not exceed 16 mg of cyproheptadine per day.
Use in children
Children from 2 to 6 years: 1 drinkable ampoule 2 times a day (equivalent to a total daily dose of 4 mg of cyproheptadine). Do not exceed 12 mg of cyproheptadine per day.
Children over 7 years and under 14: 1 drinkable ampoule 2 or 3 times a day or distributed in several administrations (equivalent to a total daily dose of 4 or 6 mg of cyproheptadine). Do not exceed 16 mg of cyproheptadine per day.
Do not exceed the recommended daily doses.
Method of administration
The ampoules should be administered preferably 30 minutes before the main meals. The contents of the ampoule can be taken alone or with water or fruit juice.
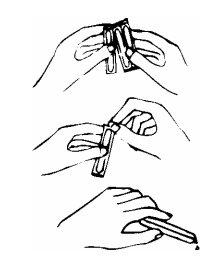
To correctly administer this medicine, follow the instructions indicated below:
top part.
ampoule and pour the contents into a glass. |
|
If you take more Dynamogén than you should
Overdose of some antihistamines in children can cause hallucinations, decreased nervous system function, convulsions, respiratory arrest, or cardiac arrest, and can even be fatal.
Consult your doctor or pharmacist immediately or go to the emergency department of the nearest hospital. Bring this leaflet with you. You can also call the Toxicology Information Service Telephone 91.562.04.20, indicating the medicine and the amount taken.
If you forget to take Dynamogén
Do not take a double dose to make up for forgotten doses.
If you stop taking Dynamogén
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Adverse reactions are, in general, mild and transient and are mainly due to cyproheptadine. Adverse reactions observed with arginine aspartate are mainly gastrointestinal. Anticholinergic effects (dry mouth, intestinal constipation, blurred vision, worsening of glaucoma) are more frequent in elderly subjects.
In general, after administration of Dynamogén, the following adverse effects have been described:
Frequent (may affect up to 1 in 10 people)
Uncommon (may affect up to 1 in 100 people)
Rare (may affect up to 1 in 1,000 people)
Very rare (may affect up to 1 in 10,000 people)
Frequency not known (cannot be estimated from the available data)
Blood and lymphatic system disorders
Frequency not known:
- Leukopenia (decrease in the number of white blood cells), neutropenia (decrease in a type of white blood cells), agranulocytosis (decrease in a type of white blood cells)
- Thrombocytopenia (decrease in the number of platelets)
- Hemolytic anemia (decrease in the number of red blood cells)
Immune system disorders
Frequency not known:
- Hypersensitivity to any of the components (tendency to present allergic reactions). Cases of asthma due to amaranth dye (E-123) included as an excipient have been described in subjects with a history of allergy to aspirin.
- Urticaria, edema.
Very rare:
- Angioedema (swelling of the face, tongue, and throat that can cause respiratory problems)
- Anaphylactic shock (severe allergic reaction)
Metabolism and nutrition disorders
Frequent:
- Increased appetite/weight gain
Psychiatric disorders
Rare:
- Excitement, nervousness, restlessness
- Mental confusion, hallucinations (visual) and irritability more frequent in cases of overdose
- Euphoria
Frequency not known:
- Insomnia, agitation, aggressive behavior
- Altered concentration (decreased concentration)
- Memory alterations (deficit).
Nervous system disorders
Frequent:
- Sedation or drowsiness, more marked at the beginning of treatment
Frequency not known:
- Motor incoordination, tremors
- Orthostatic hypotension (decrease in blood pressure when changing position)
- Paresthesia (tingling sensation, neuritis (inflammation of the nerves)
- Headache
- Convulsion (observed in children)
Eye disorders
Frequency not known:
- Mydriasis (pupil dilation)
- Alteration of visual accommodation
Ear and labyrinth disorders
Frequency not known:
- Balance disorders, vertigo more frequent in elderly patients, dizziness
- Tinnitus (ringing or buzzing in the ears)
- Vertigo
Cardiac disorders
Frequency not known:
- Palpitations
- Tachycardia (increased heart rate)
- Extrasystoles (extra heartbeats)
Respiratory, thoracic, and mediastinal disorders
Frequency not known:
- Thickening of bronchial secretions
- Dryness of mucous membranes (nasal, pharyngeal)
- Nasal congestion
Gastrointestinal disorders
Frequent:
- Nausea, vomiting, diarrhea, abdominal pain
Frequency not known:
- Dry mouth
- Epigastric pain
- Constipation
Hepatobiliary disorders
Frequency not known:
- Alteration of liver function (increase in transaminases)
- Liver failure
- Jaundice (yellowing of the skin and eyes)
- Cholestatic and/or cytolytic hepatitis
Skin and subcutaneous tissue disorders
Rare:
- Erythema (redness of the skin)
- Excessive sweating
- Photosensitivity
Renal and urinary disorders
Frequency not known:
- Urinary frequency
- Difficulty urinating
- Urinary retention
General disorders and administration site conditions
Frequency not known:
- Fatigue
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if it is possible side effects not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaRAM.es/. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Dynamogén
Keep this medicine out of the sight and reach of children.
No special storage conditions are required.
Do not use this medicine after the expiry date which is stated on the packaging after EXP. The expiry date is the last day of the month indicated.
Medicines should not be disposed of via wastewater or household waste. Return the containers and any unused medicines to the pharmacy. If you are unsure, ask your pharmacist how to dispose of the containers and any unused medicines. This will help protect the environment.
6. Contents of the pack and further information
Composition of Dynamogén 3 mg/1 g/10ml Oral Solution
- The active ingredients are arginine aspartate and ?-ketoglutarate of cyproheptadine. Each 10 ml ampoule contains 1 g of arginine aspartate and 3 mg of ?-ketoglutarate of cyproheptadine (equivalent to 2 mg of cyproheptadine).
- The other ingredients are: sorbitol (E-420) (70% solution), sucrose, potassium sorbate (E-202), raspberry flavor, hydrochloric acid, amaranth dye (E-123), and purified water.
Appearance of the product and pack contents
Dynamogén is presented in drinkable ampoules containing 10 ml of oral solution for administration by mouth. Each pack contains 20 ampoules.
Marketing authorization holder
Faes Farma, S.A.
Autonomia Etorbidea, 10
48940 Leioa (Bizkaia)
Spain
Manufacturer
Faes Farma, S.A.
Maximo Agirre Kalea, 14
48940 Leioa (Bizkaia)
Spain
O
Faes Farma, S.A.
Parque Científico y Tecnológico de Bizkaia
Ibaizabal Bidea, Edificio 901
48160 Derio (Bizkaia)
Spain
Date of last revision of this leaflet:February 2024
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to DYNAMOGÉN 3 MG/1 G/10ML ORAL SOLUTIONDosage form: ORAL SOLUTION/SUSPENSION, -Active substance: Appetite stimulantsManufacturer: Faes Farma S.A.Prescription requiredDosage form: INJECTABLE, 100 U/mlActive substance: insulin glargineManufacturer: Eli Lilly Nederland B.V.Prescription requiredDosage form: TABLET, 2 mgActive substance: loperamideManufacturer: Uxa Farma S.A.Prescription not required
Online doctors for DYNAMOGÉN 3 MG/1 G/10ML ORAL SOLUTION
Discuss questions about DYNAMOGÉN 3 MG/1 G/10ML ORAL SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions