DECAPEPTYL TRIMESTRAL 11.25 mg powder and solvent for prolonged-release injectable suspension


How to use DECAPEPTYL TRIMESTRAL 11.25 mg powder and solvent for prolonged-release injectable suspension
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Decapeptyl Trimestral 11.25 mg Powder and Solvent for Prolonged Release Suspension for Injection
Triptorelin
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Decapeptyl Trimestral and what is it used for.
- What you need to know before you use Decapeptyl Trimestral.
- How to use Decapeptyl Trimestral.
- Possible side effects.
- Storage of Decapeptyl Trimestral.
Contents of the pack and further information
1. What is Decapeptyl Trimestral and what is it used for
This medicine contains triptorelin. Triptorelin belongs to a group of medicines called gonadotropin-releasing hormone (GnRH) analogues. One of its actions is to decrease the levels of sex hormones in the body.
Decapeptyl Trimestral is indicated in adults for the treatment of hormone-dependent prostate cancer that is locally advanced or has spread to other parts of the body (metastatic cancer). It is also used to treat localized high-risk or locally advanced prostate cancer, in combination with radiotherapy.
In children, from 2 years of age, Decapeptyl Trimestral is used to treat precocious puberty that appears at a very young age, before the age of 8 in girls and 10 in boys (central precocious puberty).
2. What you need to know before you use Decapeptyl Trimestral
Do not use Decapeptyl Trimestral:
- if you are allergic (hypersensitive) to triptorelin, to gonadotropin-releasing hormone (GnRH), to other GnRH analogues or to any of the other ingredients of this medicine (listed in section 6).
- If you are pregnant or breastfeeding.
Warnings and precautions:
Consult your doctor or pharmacist before starting treatment with Decapeptyl Trimestral.
There have been reports of depression in patients treated with Decapeptyl Trimestral, which can be severe. If you are being treated with Decapeptyl Trimestral and experience depression, inform your doctor.
In adults, triptorelin may cause bones to become less dense (osteoporosis) with an increased risk of bone fractures. Therefore, inform your doctor if you have any risk factors, as they may prescribe a bisphosphonate (a medicine used to treat weak bones) to treat bone loss. Risk factors may include:
- If you or a close relative have less dense bones.
- If you drink excessive amounts of alcohol and/or are a heavy smoker.
- If you have been taking medicines that can cause your bones to become less dense for a long time, such as medicines for epilepsy or steroids (such as hydrocortisone or prednisolone).
In case of seizures, inform your doctor immediately. There have been reports of seizures in patients receiving triptorelin or similar medicines. These occurred in patients with or without a medical history of epilepsy.
If you have an unknown pituitary tumor (benign tumor), it may be discovered during treatment with Decapeptyl Trimestral. Symptoms include headache, vision problems, and paralysis of the eyes.
If you are taking medicines to prevent blood clotting, as bruising may occur at the injection site.
If you have diabetes or heart problems, inform your doctor.
In men:
- When starting treatment, the amount of testosterone in your body will increase, which can make cancer symptoms worse. Consult your doctor if this happens. Your doctor may give you a medicine (an anti-androgen) to prevent symptoms from getting worse.
- If you have urinary obstruction or spinal cord compression (nerves in your spine) due to the spread of your cancer, during the first few weeks of treatment, your doctor will closely monitor you. If you experience difficulty urinating, bone pain, weakness in your lower limbs, or a tingling sensation, consult your doctor immediately, who will evaluate and treat you accordingly.
- After surgical castration, triptorelin does not induce any further decrease in serum testosterone levels and therefore should not be used after orchiectomy (surgical removal of the testicles).
- Diagnostic tests of pituitary gonadal function or sexual organs performed during treatment or after discontinuation of treatment with Decapeptyl Trimestral may be misleading.
- If you have any problems with your blood vessels or heart, including heart rhythm problems (arrhythmia) or if you are being treated with medicines for this condition, consult your doctor. The risk of heart rhythm problems may be increased when using Decapeptyl Trimestral.
- Medicines that decrease testosterone may cause changes in the ECG associated with heart rhythm abnormalities (prolongation of the QT interval).
- Treatment with GnRH analogues such as Decapeptyl Trimestral may increase the risk of anemia (defined as a decrease in red blood cell count).
In children:
Girls with precocious puberty may experience some vaginal bleeding during the first month of treatment.
When treatment is discontinued, signs of puberty appear.
In girls, menstrual bleeding will start on average one year after treatment is discontinued.
Your doctor should rule out precocious puberty caused by other diseases.
The amount of minerals in the bones decreases during treatment but returns to normal levels after treatment is stopped.
After discontinuation of treatment, a hip disorder (slipped capital femoral epiphysis) may occur. This causes stiffness of the hip, limping, and/or severe pain in the groin that radiates to the thigh. If this happens, you should consult your doctor.
If you have a progressive brain tumor, inform your doctor. This may affect how your doctor decides to treat you.
If your child experiences severe or recurrent headache, vision problems, or ringing in the ears, contact a doctor immediately (see section 4).
Consult your doctor if you are concerned about any of these issues.
Other medicines and Decapeptyl Trimestral
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines, including those obtained without a prescription.
For men:
Decapeptyl Trimestral may interfere with some medicines used to treat heart rhythm problems (e.g., quinidine, procainamide, amiodarone, and sotalol) or may increase the risk of heart rhythm problems when used with other medicines (e.g., methadone (used for pain relief and as part of drug detoxification), moxifloxacin (an antibiotic), antipsychotics used for severe mental illnesses).
Pregnancy and breastfeeding
Decapeptyl Trimestral should not be used during pregnancy or breastfeeding.
.
Driving and using machines
You may feel dizzy, tired, or have vision problems, such as blurred vision. These are possible side effects of treatment or due to the underlying disease. If you experience any of these side effects, do not drive or operate machinery.
Decapeptyl Trimestral contains sodium
This medicine contains sodium but less than 1 mmol of sodium (23 mg) per vial, i.e., "sodium-free).
3. How to use Decapeptyl Trimestral
Follow the instructions for administration of this medicine exactly as indicated by your doctor. In case of doubt, consult your doctor or pharmacist again.
Decapeptyl Trimestral should be administered exclusively by intramuscular injection. Your doctor or nurse will administer it. See the Instructions for use at the end of this leaflet.
For localized high-risk or locally advanced prostate cancer, in combination with radiotherapy, the recommended treatment duration is 2-3 years.
The dose will be determined by your doctor based on the needs of each patient. The usual doses are as follows:
Prostate cancer: One deep intramuscular injection of Decapeptyl Trimestral every three months.
Use in children:
Normally, you will receive an injection every 3 months. Decapeptyl Trimestral is only for injection into the muscle. Your doctor will decide when treatment should be discontinued (usually when you are 12-13 years old, in the case of girls, and 13-14 years old in boys).
If you feel that the effect of Decapeptyl Trimestral is too strong or too weak, tell your doctor or pharmacist.
If you use more Decapeptyl Trimestral than you should
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately.
If you forget to use Decapeptyl Trimestral
As soon as you realize you have missed an injection, consult your doctor, and they will decide when you should have the next injection.
If you stop using Decapeptyl Trimestral
Do not stop treatment with Decapeptyl Trimestral without talking to your doctor first.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
In rare cases, you may experience a severe allergic reaction (angioedema, anaphylactic reaction). Inform your doctor immediately if you develop symptoms such as difficulty swallowing or breathing, dizziness, swelling of the lips, face, throat, or tongue, or a rash.
If you present an unknown pituitary tumor (benign tumor), it may be discovered during treatment with Decapeptyl Trimestral. Symptoms include sudden headache, vision problems, and paralysis of the eyes.
As with other GnRH analogues, an increase in white blood cell count may occur in patients treated with Decapeptyl Trimestral.
In men
Many of these side effects are expected due to the change in testosterone levels in your body. These effects include hot flashes, impotence, and decreased libido.
With the exception of immune-allergic reactions and reactions at the injection site, all side effects are related to changes in testosterone levels.
Very common side effects(may affect more than 1 in 10 patients):
- Hot flashes
- Weakness
- Excessive sweating
- Back pain
- Tingling and numbness in the legs
- Decreased libido
- Impotence
Common side effects(may affect 1 in 10 patients):
- Nausea, dry mouth
- Pain, bruising, redness, and swelling at the injection site, edema (fluid accumulation in body tissues),
- Muscle and bone pain, pain in arms and legs
- Lower abdominal pain
- Allergic reaction
- Weight gain
- Dizziness, headache
- Loss of libido, depression, mood changes
- High blood pressure
Uncommon side effects(may affect 1 in 100 patients):
- Increased platelet count
- Feeling of heartbeats
- Ringing in the ears, vertigo,
- Blurred vision
- Abdominal pain, constipation, diarrhea, vomiting
- Drowsiness, intense shivering associated with sweating and fever, somnolence, pain
- Certain blood test parameters affected (including increased liver function tests), increased blood pressure
- Weight loss
- Loss of appetite, increased appetite, gout (severe pain and swelling of the joints, usually in the big toe), diabetes, high blood lipid levels
- Joint pain, muscle cramps, muscle weakness, muscle pain, swelling of the ankles, feet, or fingers, bone pain
- Tingling or numbness
- Insomnia, irritability
- Waking up to urinate, problems urinating
- Breast development in men, chest pain, reduced testicular size, testicular pain
- Breathing difficulties,
- Nosebleeds
- Acne, hair loss, itching, rash, redness of the skin, hives
Rare side effects(may affect 1 in 1,000 patients):
- Purple or red discoloration of the skin
- Abnormal sensation in the eye, vision changes or blurred vision
- Feeling of fullness in the abdomen, flatulence, dry mouth, abnormal taste
- Chest pain
- Difficulty standing
- Flu-like symptoms, fever
- Severe allergic reaction that can cause dizziness or difficulty breathing, swelling of the face, throat, or tongue)
- Nasal/throat inflammation
- Increased body temperature
- Joint stiffness, joint swelling, musculoskeletal stiffness, osteoarthritis (joint disorder that affects cartilage and causes pain, swelling, and loss of movement)
- Memory loss
- Feeling of confusion, decreased activity, feeling of euphoria
- Difficulty breathing when lying down
- Blisters
- Low blood pressure
During post-marketing experience, the following side effects have also been reported:
- Severe allergic reaction that can cause difficulty breathing or dizziness and swelling of the face, neck, or throat (angioedema, anaphylactic shock)
- Changes in the ECG (prolongation of the QT interval)
- General malaise
- Anxiety
- Rapid formation of papules due to swelling of the skin or mucous membranes,
- Urinary incontinence
- If there is a pituitary tumor, there is a higher risk of bleeding in the area
- Anemia (decrease in red blood cell count)
Your doctor will determine the measures to be taken to counteract them.
Reporting of side effects
If you experience any side effects, talk to your doctor, even if it is possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines. www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Decapeptyl Trimestral
Keep this medicine out of the sight and reach of children.
Store Decapeptyl Trimestral in its original package. Do not store above 25°C.
Do not use Decapeptyl Trimestral after the expiry date stated on the package after EXP. The expiry date is the last day of the month indicated.
Medicines should not be disposed of via wastewater or household waste. Place the packaging and any unused medicine in the SIGRE collection point at the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Container Content and Additional Information
Composition of Decapeptyl Trimestral
The active ingredient is triptorelin (pamoate), 11.25 mg per vial.
The other components are:
- Powder: DL lactide-co-glycolide polymer, mannitol, sodium carmellose, polysorbate 80
- Solvent: mannitol and water for injectable preparations.
Appearance of the Product and Container Content
This medication is a powder and solvent for injectable suspension, the powder is a slightly yellowish lyophilized powder and the solvent for the reconstitution of the suspension is a clear, colorless solution.
Container with 1 vial, 1 ampoule, and 1 blister with 1 syringe and 2 needles for reconstitution and administration.
Marketing Authorization Holder and Manufacturer
Marketing authorization holder:
IPSEN PHARMA, S.A.U.
Gran Via de les Corts Catalanes 130-136
08038 Barcelona
Spain
Manufacturer:
IPSEN PHARMA-BIOTECH
Parc d'Activité du Plateau de Signes, C.D. 402,
83870 Signes
France
Date of the Last Revision of thisProspectus:February 2025
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es. This information is intended only for healthcare professionals: INSTRUCTIONS FOR RECONSTITUTION
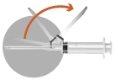
1 –PATIENT PREPARATION BEFORE RECONSTITUTION | |
| |
2 – PREPARATION OF THE INJECTION | |
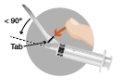
The box includes two needles:
| |
The presence of bubbles in the upper part of the lyophilized powder is a normal aspect of the product. The following steps must be completed in a continuous sequence. | |
2a
|
|
2b
|
|
2c
|
|
2d
|
|
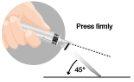
3 –INTRAMUSCULAR INJECTION | |
|
|
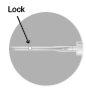
4 –AFTER USE | |
There are two alternative methods to activate the safety system.
Used needles, any unused suspension, or other residual material must be discarded in accordance with local guidelines. |
Method A
Method B
|
Any incident that causes significant loss of the product to be injected must be reported to the doctor, who will determine the convenience of repeating the injection in a shorter period.
|
TREATMENT TABLE
Surname
Name
Date of Request | Date of Injection | |
Injection 1 | ||
Injection 2 | ||
Injection 3 | ||
Injection 4 | ||
Injection 5 | ||
Injection 6 |
- Country of registration
- Average pharmacy price308.66 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to DECAPEPTYL TRIMESTRAL 11.25 mg powder and solvent for prolonged-release injectable suspensionDosage form: INJECTABLE, 0.1 mgActive substance: triptorelinManufacturer: Ipsen Pharma S.A.Prescription requiredDosage form: INJECTABLE, 3.75 mg triptorelin/vialActive substance: triptorelinManufacturer: Ipsen Pharma S.A.Prescription requiredDosage form: INJECTABLE, 22.5 mgActive substance: triptorelinManufacturer: Ipsen Pharma S.A.U.Prescription required
Online doctors for DECAPEPTYL TRIMESTRAL 11.25 mg powder and solvent for prolonged-release injectable suspension
Discuss questions about DECAPEPTYL TRIMESTRAL 11.25 mg powder and solvent for prolonged-release injectable suspension, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions