CETRAXAL PLUS 3 mg/ml + 0.25 mg/ml OTIC SOLUTION DROPS

How to use CETRAXAL PLUS 3 mg/ml + 0.25 mg/ml OTIC SOLUTION DROPS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Cetraxal plus 3 mg/ml + 0.25 mg/ml ear drops in solution
Ciprofloxacin / Fluocinolone Acetonide
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Cetraxal plus is and what it is used for.
- What you need to know before you use Cetraxal plus.
- How to use Cetraxal plus.
- Possible side effects.
- Storage of Cetraxal plus.
- Contents of the pack and other information.
1. What is Cetraxal Plus and what is it used for
Cetraxal Plus is a solution for otic use (in the ear). It contains:
- Ciprofloxacin, which belongs to a group of antibiotics called fluoroquinolones.
Ciprofloxacin acts by eliminating bacteria that cause infections,
- and fluocinolone acetonide, a corticosteroid with anti-inflammatory and analgesic properties for the treatment of inflammation and pain.
Antibiotics are used to treat bacterial infections and are not effective against viral infections such as the flu or the common cold. It is essential that you follow the instructions regarding dosage, administration interval, and treatment duration indicated by your doctor. Do not store or reuse this medication. If you have any leftover antibiotic after completing the treatment, return it to the pharmacy for proper disposal. Do not throw away medications down the drain or in the trash. |
Cetraxal Plus is an ear drop solution. It is used in adults and children aged 6 months and older to treat acute external otitis (infection of the outer ear) and otitis media (infection of the middle ear) with tympanostomy tubes (ear tubes) of bacterial origin.
You should consult a doctor if your symptoms worsen or do not improve after completing the treatment.
2. What you need to know before you use Cetraxal Plus
Do not use Cetraxal Plus:
- If you are allergic to ciprofloxacin, other quinolones, fluocinolone acetonide, or any of the other components of this medication (listed in section 6).
- If you have a viral or fungal ear infection.
Warnings and precautions
- This medication should only be applied in the ear. It should not be ingested, injected, or inhaled. It should not be applied to the eye.
- If you experience symptoms of urticaria (itching) or skin rash or any other allergic symptom (such as sudden swelling of the face, throat, or eyelids, difficulty breathing) after starting treatment, discontinue the medication immediately and consult your doctor. Severe hypersensitivity reactions may require emergency treatment.
If your symptoms do not improve before completing the treatment, talk to your doctor.
- As with other antibiotics, infections can sometimes be caused by organisms that are not sensitive to ciprofloxacin. In such cases, the appropriate treatment should be prescribed by your doctor.
- Contact your doctor if you experience blurred vision or other visual disturbances.
Children:
There is not enough clinical experience with the use of Cetraxal Plus in children under 6 months, so you should consult your doctor before administering this medication to your child if they are of this age.
Use of Cetraxal Plus with other medications:
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medications, including those obtained without a prescription.
The use of Cetraxal Plus with other ear medications is not recommended.
Pregnancy, breastfeeding, and fertility
There are no adequate and well-controlled studies in pregnant women on the teratogenic effects of fluocinolone acetonide.
If you are pregnant or breastfeeding, or think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medication.
Caution should be exercised when administering Cetraxal Plus to a breastfeeding woman since it is not known whether Cetraxal Plus is excreted in breast milk.
Driving and using machines:
Given the characteristics and route of administration of the medication, Cetraxal Plus does not affect the ability to drive vehicles or operate hazardous machinery.
Cetraxal Plus contains:
Cetraxal Plus may cause allergic reactions (possibly delayed) because it contains methyl parahydroxybenzoate and propyl parahydroxybenzoate.
3. How to use Cetraxal Plus
Cetraxal Plus is intended for otic use only (in the ear). Follow your doctor's instructions for administering this medication exactly. If in doubt, consult your doctor or pharmacist again.
The recommended dose in adults and children is 6 to 8 drops twice a day in the affected ear for 7 days.
Only administer Cetraxal Plus in both ears if your doctor recommends it.
Your doctor will indicate the duration of treatment with Cetraxal Plus. To ensure that the infection does not recur, do not interrupt treatment prematurely, even if you notice improvement in the ear(s).
Administration instructions:
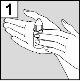
The person administering Cetraxal Plus should wash their hands beforehand.
|
|
|
|
|
|
|
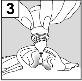
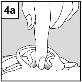
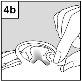
For patients with middle ear infection with drainage tubes: While the patient remains lying on their side, the person administering Cetraxal Plus should gently press the skin fold at the beginning of the ear canal 4 timeswith a pumping motion. This will allow the drops to pass through the eardrum and reach the middle ear. For patients with external ear infection: While the patient remains lying on their side, the person administering Cetraxal Plus should gently pull the earlobe forward and backward. This will allow the drops to penetrate the ear canal. |
- Keep your head tilted towards the affected ear for approximately one minute to allow the medication to enter the ear.
- Repeat the operation, if necessary, in the other ear.
It is essential that you follow these instructions to achieve a good result with this medication in your ear. Once the medication has been administered in the ear, keeping your head in a vertical position or moving your head too quickly may cause some of the medication to be lost because the drops may fall down your face and not penetrate the inner part of the ear canal.
Keep the bottle until the end of the treatment. Do not store it for later use.
If you use more Cetraxal Plus than you should
The symptoms related to overdose are not known.
In case of overdose or accidental ingestion, inform your doctor or pharmacist or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medication and the amount ingested.
If you forget to use Cetraxal Plus
Do not use a double dose to make up for forgotten doses. Simply continue with your next dose.
If you stop using Cetraxal Plus
Do not stop using Cetraxal Plus without consulting your doctor or pharmacist. It is very important to use these ear drops for the time your doctor has indicated, even if your symptoms improve. If you stop using this medication too soon, the infection may not disappear, and your symptoms may recur or even worsen. Resistance to antibiotics may also occur.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Cetraxal plus can cause side effects, although not everybody gets them.
If you experience a severe allergic reaction and any of the following symptoms appear, discontinue treatment with this medication and consult your doctor immediately: swelling of hands, feet, ankles, face, lips, mouth, or throat, difficulty swallowing or breathing, rash, or hives.
Common: may affect up to 1 in 10 people
Ear effects:Discomfort, pain, itching.
General side effects:Alteration in taste.
Uncommon: may affect up to 1 in 100 people
Ear effects: Ringing, residue of medication, blockage of the ear drainage tube, tingling, congestion, reduced hearing, skin rash, redness, external ear fungal infection, discharge, inflammation, eardrum disorder, granulation tissue, otitis media in the other ear.
General side effects:Fungal infection (Candida), irritability, crying, dizziness, flushing, headache, vomiting, fatigue.
Frequency not known (cannot be estimated from the available data):Blurred vision.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Medicines and Health Products Agency's website: www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medication.
5. Storage of Cetraxal plus
Keep this medication out of the sight and reach of children.
Store below 30°C.
Do not use this medication after the expiry date stated on the packaging after "EXP". The expiry date is the last day of the month indicated.
This medication should be discarded one month after opening. Once opened, store below 25°C.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
Composition of Cetraxal Plus
- The active ingredients are ciprofloxacin (as hydrochloride) and fluocinolone acetonide. Each milliliter of Cetraxal Plus contains 3 mg of ciprofloxacin (as hydrochloride) and 0.25 mg of fluocinolone acetonide.
- The other ingredients are methyl parahydroxybenzoate (E218), propyl parahydroxybenzoate (E216), povidone, diethylene glycol monoethyl ether, glycereth-26 (a compound of glycerin and ethylene oxide), hydrochloric acid (E507)/sodium hydroxide (E524), and purified water.
Appearance and packaging of the product
Cetraxal Plus is a clear solution in the form of ear drops. It comes in white opaque plastic bottles with a dropper. Each pack contains 10 ml of solution.
Marketing authorization holder and
Laboratorios SALVAT, S.A.
C/ Gall 30-36
08950 - Esplugues de Llobregat
Barcelona (Spain)
Manufacturer
Laboratorios SALVAT, S.A.
C/ Gall 30-36
08950 - Esplugues de Llobregat
Barcelona (Spain)
or
PHARMALOOP S.L.
C/Bolivia, 15 - Polig. Industrial Azque
28806 Alcala de Henares, Madrid
Spain
This leaflet was approved in June 2019.
This medication is authorized in the Member States of the European Economic Area under the following names:
Austria InfectoCiproCort 3 mg/ml + 0.25 mg/ml Ohrentropfen, Lösung
Germany InfectoCiproCort 3 mg/ml + 0.25 mg/ml Ohrentropfen, Lösung
Denmark Cetraxal Comp 3 mg/ml + 0.25 mg/ml Øredråber, opløsning
Slovakia Infalin duo 3 mg/ml + 0.25 mg/ml Ušná roztoková instilácia
Spain Cetraxal Plus 3 mg/ml + 0.25 mg/ml ear drops in solution
France CETRAXAL 3mg + 0.25mg par ml, solution pour instillation auriculaire
Finland Cetraxal Comp 3 mg/ml + 0.25 mg/ml Korvatipat, liuos
Iceland Cetraxal Comp 3 mg/ml + 0.25 mg/ml Eyrnadropar, lausn
Norway Cetraxal Comp
Poland Cetraxal plus
Portugal Cetraxal Duo 3 mg/ml + 0.25 mg/ml ear drops, solution
Czech Republic Infalin duo 3 mg/ml + 0.25 mg/ml ušní kapky, roztok
Romania Cexidal 3 mg/ml + 0.25 mg/ml Picaturi auriculare, solutie
Sweden Cetraxal Comp 3 mg/ml + 0.25 mg/ml Örondroppar, lösning
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS)
http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price7.91 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CETRAXAL PLUS 3 mg/ml + 0.25 mg/ml OTIC SOLUTION DROPSDosage form: OTIC SOLUTION, 0.25 mg / 3.49 mgActive substance: fluocinolone acetonide and antiinfectivesManufacturer: Zambon S.A.U.Prescription requiredDosage form: OTIC SOLUTION, 3 mg/ml + 0.25 mg/mlActive substance: fluocinolone acetonide and antiinfectivesManufacturer: Laboratorios Salvat S.A.Prescription requiredDosage form: OTIC SOLUTION, 0.25 - 3 mg/mlActive substance: fluocinolone acetonide and antiinfectivesManufacturer: Industrial Farmaceutica Cantabria S.A.Prescription required
Online doctors for CETRAXAL PLUS 3 mg/ml + 0.25 mg/ml OTIC SOLUTION DROPS
Discuss questions about CETRAXAL PLUS 3 mg/ml + 0.25 mg/ml OTIC SOLUTION DROPS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions