ACEOTO PLUS 3 mg/ml + 0.25 mg/ml OTIC SOLUTION DROPS

How to use ACEOTO PLUS 3 mg/ml + 0.25 mg/ml OTIC SOLUTION DROPS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
PACKAGE LEAFLET: INFORMATION FOR THE USER
Aceoto Plus 3 mg/ml + 0.25 mg/ml ear drops in solution
Ciprofloxacin / Fluocinolone Acetonide
Read the entire package leaflet carefully before starting to use this medicine, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, ask your doctor or pharmacist.
- This medicine has been prescribed to you and should not be given to others, even if they have the same symptoms, as it may harm them.
If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. See section 4.
Contents of the package leaflet
- What is Aceoto Plus and what is it used for
- What you need to know before using Aceoto Plus
- How to use Aceoto Plus
- Possible side effects
- Storage of Aceoto Plus
- Package contents and additional information
1. What is Aceoto Plus and what is it used for
Aceoto Plus is a solution for otic use (in the ear).
It contains:
- Ciprofloxacin, which belongs to the group of antibiotics called fluoroquinolones.
Ciprofloxacin acts by eliminating bacteria that cause infections,
- and fluocinolone acetonide, a corticosteroid with anti-inflammatory and analgesic properties for the treatment of inflammation and pain.
Antibiotics are used to treat bacterial infections and are not effective against viral infections such as the flu or common cold. It is essential to follow the instructions regarding dosage, administration interval, and treatment duration indicated by your doctor. Do not store or reuse this medicine. If you have any leftover antibiotic after completing the treatment, return it to the pharmacy for proper disposal. Do not throw medicines down the drain or in the trash. |
Aceoto Plus is an ear drop solution. It is used in adults and children aged 6 months and older to treat acute external otitis (outer ear infection) and otitis media (middle ear infection) with tympanostomy tubes (drainage tubes) of bacterial origin.
You should consult a doctor if your symptoms worsen or do not improve after completing the treatment.
2. What you need to know before using Aceoto Plus
Do not use Aceoto Plus
- If you are allergic to ciprofloxacin, other quinolones, fluocinolone acetonide, or any of the other components of this medicine (listed in section 6).
- If you have a viral or fungal ear infection.
Warnings and precautions
- This medicine should only be applied in the ear. It should not be ingested, injected, or inhaled. It should not be applied to the eye.
- If you experience symptoms of urticaria (itching) or skin rash or any other allergic symptom (such as sudden swelling of the face, throat, or eyelids, difficulty breathing) after starting treatment, discontinue the medication and consult your doctor immediately. Severe hypersensitivity reactions may require immediate emergency treatment.
- Tell your doctor if your symptoms do not improve before completing the treatment.
As with other antibiotics, infections may sometimes be caused by organisms that are not susceptible to ciprofloxacin. In such cases, your doctor should prescribe the appropriate treatment.
- Contact your doctor if you experience blurred vision or other visual disturbances.
Use in children
Children: There is insufficient clinical experience with the use of Aceoto Plus in children under 6 months of age. You should consult your doctor before administering this medicine to your child if they are of this age.
Use of Aceoto Plus with other medicines
Tell your doctor or pharmacist if you are taking/using, have recently taken/used, or might take/use any other medicine, including those obtained without a prescription.
The use of Aceoto Plus with other ear medicines is not recommended.
Pregnancy, breastfeeding, and fertility
There are no adequate and well-controlled studies in pregnant women on the teratogenic effects of fluocinolone acetonide.
If you are pregnant, breastfeeding, or think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Caution should be exercised when administering Aceoto Plus to a breastfeeding woman, as it is not known whether Aceoto Plus is excreted in breast milk.
Driving and using machines
Given the characteristics and route of administration of the medicine, Aceoto Plus does not affect the ability to drive vehicles or operate hazardous machinery.
Aceoto Plus containsMethyl Parahydroxybenzoate and Propyl Parahydroxybenzoate
Aceoto Plus may cause allergic reactions (possibly delayed) because it contains methyl parahydroxybenzoate and propyl parahydroxybenzoate.
3. How to use Aceoto Plus
Aceoto Plus is intended for otic use only (in the ear). Follow your doctor's instructions for administering this medicine exactly. If in doubt, consult your doctor or pharmacist again.
The recommended dose in adults and children is 6 to 8 drops twice a day in the affected ear for 7 days.
Only administer Aceoto Plus in both ears if your doctor recommends it.
Your doctor will indicate the duration of treatment with Aceoto Plus. To ensure that the infection does not recur, do not interrupt treatment prematurely, even if you notice improvement in the ear(s).
Administration instructions
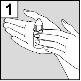
The person administering Aceoto Plus should wash their hands beforehand.
|
|
|
|
|
|
|
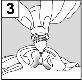
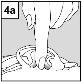
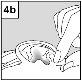
For patients with middle ear infection with drainage tubes: While the patient remains lying on their side, the person administering Aceoto Plus should gently press the skin fold at the beginning of the ear canal (figure 4a) 4 timeswith a pumping motion. This will allow the drops to pass through the eardrum and reach the middle ear. For patients with external ear infection: While the patient remains lying on their side, the person administering Aceoto Plus should gently pull the earlobe forward and backward (figure 4b). This will allow the drops to enter the ear canal. |
- Keep your head tilted to the side of the affected ear for about one minute to allow the medicine to enter the ear.
- Repeat the operation, if necessary, in the other ear.
It is essential to follow these instructions to achieve a good result with this medicine in your ear. Once the medicine has been administered in the ear, keeping your head in a vertical position or moving your head too quickly may cause some of the medication to be lost because the drops may fall down your face and not penetrate the inner part of the ear canal.
Keep the bottle until the end of the treatment. Do not store it for later use.
If you use more Aceoto Plus than you should
The symptoms related to overdose are not known. In case of overdose or accidental ingestion, inform your doctor or pharmacist or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount ingested.
If you forget to use Aceoto Plus
Do not use a double dose to make up for forgotten doses. Simply continue with your next dose.
If you stop using Aceoto Plus
Do not stop using Aceoto Plus without consulting your doctor or pharmacist. It is crucial to use these ear drops for the time your doctor has indicated, even if your symptoms improve. If you stop using this medicine prematurely, the infection may not disappear, and your symptoms may recur or worsen. Antibiotic resistance may also occur.
If you have any further questions about the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Aceoto Plus can cause side effects, although not everyone will experience them.
If you experience a severe allergic reaction and any of the following symptoms appear, discontinue treatment with this medicine and consult your doctor immediately: swelling of hands, feet, ankles, face, lips, mouth, or throat, difficulty swallowing or breathing, rash, or hives, ulcers.
Common: may affect up to 1 in 10 people
Ear effects:Discomfort, pain, itching.
General side effects:Alteration in taste.
Uncommon: may affect up to 1 in 100 people
Ear effects:Ringing, residual medication, blockage of the ear drainage tube, tingling, congestion, reduced hearing, skin rash, redness, external ear fungal infection, discharge, inflammation, eardrum disorder, granulation tissue, otitis media in the other ear.
General side effects:Fungal infection (Candida), irritability, crying, dizziness, flushing, headache, vomiting, fatigue.
Frequency not known (cannot be estimated from available data):Blurred vision.
Reporting side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. You can also report them directly through the Spanish Medicines Monitoring System: www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Aceoto Plus
Keep this medicine out of the sight and reach of children.
Store below 30°C.
Do not use this medicine after the expiration date stated on the packaging after "EXP". The expiration date is the last day of the month indicated.
This medicine should be discarded one month after opening.
Medicines should not be thrown down the drain or into the trash. Deposit the packaging and any unused medicines at the pharmacy's SIGRE point. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medicines. This will help protect the environment.
6. Package contents and additional information
Composition of Aceoto Plus
The active ingredients are ciprofloxacin (as hydrochloride) and fluocinolone acetonide. Each milliliter of Aceoto Plus contains 3 mg of ciprofloxacin (as hydrochloride) and 0.25 mg of fluocinolone acetonide.
The other ingredients are methyl parahydroxybenzoate (E218), propyl parahydroxybenzoate (E216), povidone-K-90-F, diethylene glycol monoethyl ether, glycereth-26 (a compound of glycerin and ethylene oxide), hydrochloric acid (E507)/sodium hydroxide (E524), and purified water.
Appearance of the product and package contents
Aceoto Plus is a colorless or slightly yellowish aqueous solution in the form of ear drops. It is presented in white opaque plastic bottles with a dropper. Each package contains 10 ml of solution.
Marketing authorization holder and manufacturer
Marketing authorization holder
Zambon S.A.U.
Maresme, 5. Pol. Can Bernades-Subirà
08130 - Santa Perpètua de Mogoda
Barcelona (Spain)
Manufacturer
Laboratorios Salvat, S.A.
C/ Gall 30-36
08950 - Esplugues de Llobregat
Barcelona (Spain)
or
Pharmaloop, S.L.
C/Bolivia, 15 – Polig.Industrial Azque
28806 Alcalá de Henares,
Madrid (Spain)
This package leaflet was approved in November 2020.
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/.
- Country of registration
- Average pharmacy price7.91 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ACEOTO PLUS 3 mg/ml + 0.25 mg/ml OTIC SOLUTION DROPSDosage form: OTIC SOLUTION, 3 mg/ml + 0.25 mg/mlActive substance: fluocinolone acetonide and antiinfectivesManufacturer: Laboratorios Salvat S.A.Prescription requiredDosage form: OTIC SOLUTION, 3mg ciprofloxacin HCl; 0.25mg fluocinolone acetonideActive substance: fluocinolone acetonide and antiinfectivesManufacturer: Laboratorios Salvat S.A.Prescription requiredDosage form: OTIC SOLUTION, 0.25 - 3 mg/mlActive substance: fluocinolone acetonide and antiinfectivesManufacturer: Industrial Farmaceutica Cantabria S.A.Prescription required
Online doctors for ACEOTO PLUS 3 mg/ml + 0.25 mg/ml OTIC SOLUTION DROPS
Discuss questions about ACEOTO PLUS 3 mg/ml + 0.25 mg/ml OTIC SOLUTION DROPS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions