
CAYSTON 75 mg POWDER AND SOLVENT FOR SOLUTION FOR NEBULIZER INHALATION

How to use CAYSTON 75 mg POWDER AND SOLVENT FOR SOLUTION FOR NEBULIZER INHALATION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Cayston 75 mg powder and solvent for solution for inhalation by nebulizer
Aztreonam
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Cayston and what is it used for
- What you need to know before you use Cayston
- How to use Cayston
- Possible side effects
- Storing Cayston
- Contents of the pack and other information
1. What is Cayston and what is it used for
Cayston contains the active substance aztreonam. Cayston is an antibiotic that is used to treat long-term lung infections caused by the bacterium Pseudomonas aeruginosa in patients aged 6 years and older with cystic fibrosis. Cystic fibrosis, also known as mucoviscidosis, is a life-threatening inherited disease that affects the mucous glands of the internal organs, particularly the lungs, but also the liver, pancreas, and digestive system. In the lungs, cystic fibrosis causes congestion with sticky mucus. This produces difficulty breathing.
2. What you need to know before you use Cayston
Do not use Cayston
- if you are allergic to aztreonam or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor before starting treatment with Cayston:
- if you are allergic to any other antibiotic(such as penicillins, cephalosporins, and/or carbapenems)
- if you do not tolerate or feel chest tightness when taking other inhaled medicines
- if you have kidney problems
- if you have ever coughed up blood
- if you have ever had low lung function test results.
If you are in any of the above situations, tell your doctorbefore using Cayston.
Since it is an inhaled medicine, Cayston may cause you to cough, which could lead to coughing up blood. If you have ever coughed up blood, you should only use Cayston if your doctor believes that the benefit of taking this medicine outweighs the risk of coughing up blood.
During treatment with Cayston, you may experience a temporary decrease in lung function tests, but this is usually not a lasting effect.
Children
Cayston must not be given to children under 6 years of age.
Other medicines and Cayston
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before taking this medicine.
There are no clinical data on the use of Cayston in pregnant women, therefore, you should not use Cayston during pregnancy unless you have discussed this with your doctor.
If you are breast-feeding, ask your doctor for advice before taking Cayston. You can breast-feed while being treated with Cayston, as the amount of medicine that will pass to your baby during breast-feeding will be extremely small.
Driving and using machines
Cayston is unlikely to affect your ability to drive or use machines.
3. How to use Cayston
Follow the instructions for administration of this medicine exactly as told by your doctor. If you are unsure, consult your doctor or pharmacist again.
The recommended dose is:
- Use Cayston 3 times a day in repeated cycles of 28 days of therapy followed by 28 days without therapy with Cayston.Each of the three doses should be administered by inhalation with an interval of at least 4 hours, using the Altera handheld nebulizer device. You can use an eBase Controller or an eFlow rapid control unit with the Altera handheld device.
- Each dose consists of a vial of Cayston mixed with the contents of the solvent ampoule. It is necessary to mix Cayston with a solvent before inhaling it using the Altera nebulizer.
Insert the prepared Cayston solution into the Altera handheld nebulizer device (see below). Each treatment takes around 2 to 3 minutes to inhale.
Use a bronchodilator before each dose of Cayston. Short-acting bronchodilators can be used between 15 minutes and 4 hours before each dose of Cayston, and long-acting ones between 30 minutes and 12 hours before.
If you are using other inhaled therapies to treat cystic fibrosis, the recommended order of use is as follows:
- bronchodilator
- mucolytics (a medicine that helps dissolve the thick mucus produced in the lungs) and finally:
- Cayston.
Do not mix Cayston with any other medicinein the Altera handheld nebulizer device.
- Do not insert other medicines into the Altera handheld nebulizer device.
- Do not insert aztreonam for intravenous administration (injectable) into the Altera handheld nebulizer device. Intravenous aztreonam is not suitable for inhalation.
How to administer Cayston using the Altera handheld nebulizer device
You will need the following:
- A brown glass vial of Cayston with a blue closure cap.
- A plastic ampoule of solvent (0.17% w/v sodium chloride). The information on the solvent ampoule is presented in English only (see section 6).
- An Altera handheld nebulizer device with an Altera aerosol generator connected to an eFlow control unit of type 178 (eFlow rapid) or type 678 (eBase Controller).
You must use the Cayston-specific Altera handheld nebulizer device with the Altera aerosol generator. Do not attempt to use Cayston with any other type of handheld nebulizer device (even the eFlow rapid handheld device).
Check that the nebulizer is working properlybefore starting treatment with Cayston. Read the manufacturer's instructions for use carefully, which are supplied with the Altera nebulizer system.
Preparing Cayston for inhalation
- Do not prepare Cayston until you are ready to administer the dose.
- Do not use Cayston if you notice that the packaging has been tampered with.
- Do not use Cayston if it has been stored outside the refrigerator for more than 28 days.
- Do not use the solvent or prepared Cayston if it appears cloudy or particles are visible in the solution.
- Remove a brown glass vial of Cayston and a solvent ampoulefrom the box. The solvent ampoules should be separated by gently pulling them apart.
- Gently tap the brown glass vialof Cayston so that the powder settles at the bottom. This helps ensure that the administered dose is correct.
- Follow steps A to D in Figure 1 below to open the brown glass vial:
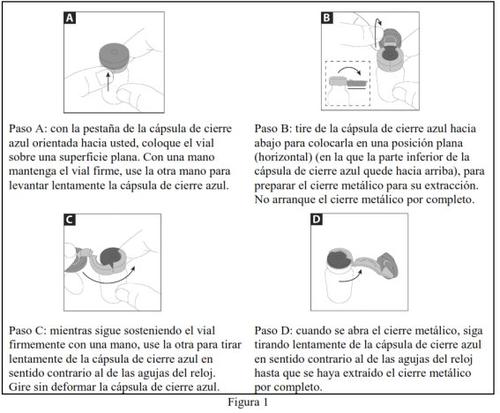
- Discard the metal closure safely. Carefully remove (but do not discard yet) the rubber stopper.
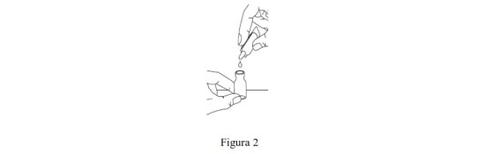
- Open the solvent ampoule by twisting the tip until it comes off. Squeeze the ampoule to transfer its contents to the vial (Figure 2). Then, gently shake the vial in a circular motion until the powder is completely dissolved and the liquid has a clear appearance.
It is best to use Cayston immediately after preparing the solution.But if you cannot use the prepared dose immediately, replace the stopper on the vial and store it in the refrigerator. Use the prepared solution within a maximum of 8 hours.
Preparing the Altera nebulizer for administration of Cayston
- Make sure the Altera handheld nebulizer deviceis placed on a smooth and stable surface.
- Remove the cap from the medicine containerby turning it counterclockwise.
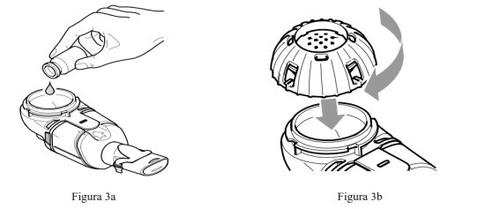
- Pour all the prepared Caystoninto the medicine container of the Altera handheld nebulizer device (Figure 3a). Make sure to empty the vial completely. Gently tap the vial against the side of the medicine container if necessary.
- Close the medicine containerby aligning the guides on the cap with the slots on the container. Press down and turn the cap clockwise until it reaches the stop (Figure 3b).
Using the Altera nebulizer for administration of Cayston
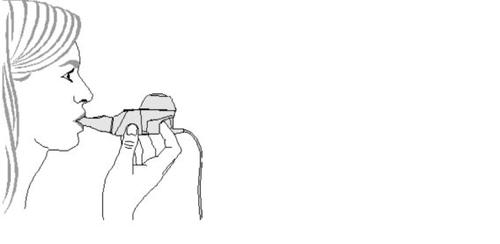
- Start treatment.Sit in a relaxed position, with your back straight. Hold the handheld device horizontally, insert the mouthpiece into your mouth, and close your lips around it (Figure 4).
Keep the handheld device in a horizontal position.
- Press and hold the On/Off buttonon the control unit for a few seconds. You will hear a beep and the status light will turn green.
- After a few seconds, a mist-like aerosol will start flowing into the nebulizer chamber of the Altera handheld nebulizer device. If the mist does not start flowing, consult the Altera system manual for information.
- Breathe normally(inhale and exhale) through the mouthpiece. Avoid breathing through your nose. Continue inhaling and exhaling comfortably until the treatment is complete.
- When all the medicine has been delivered, you will hear a sound that means "treatment complete" (2 beeps).
- When the treatment is complete, open the cap of the medicine container to ensure that it has been used up. Some drops of medicine may remain in the container after the treatment is finished. If there are more than a few drops of liquid left, replace the cap on the medicine container and restart the treatment.
- Once the treatment is complete, disconnect the control unit and set aside the Altera handheld nebulizer device to clean and disinfect it. For detailed information on cleaning and disinfection procedures, consult the manufacturer's instructions for use supplied with the Altera handheld nebulizer device.
What if I need to interrupt treatment before it's finished?
- If, for any reason, you need to interrupt treatment before it's finished, press and hold the On/Off button for a full second. To restart treatment, press and hold the On/Off button for a full second and restart the treatment.
Replacing the Altera handheld nebulizer device
The Altera handheld nebulizer device is designed to last for three cycles of 28 days of treatment with Cayston when used according to the instructions provided. After this time, replace your Altera handheld nebulizer device, including the aerosol generator. If you notice that its performance has changed before this time (for example, if it takes longer to generate the mist, more than five minutes), consult the Altera nebulizer instructions.
If you use more Cayston than you should
If you have used more Cayston than you should, consult a doctor or pharmacist immediately.
If you forget to use Cayston
If you miss a dose, you can continue to administer the 3 daily doses as long as you leave an interval of at least 4 hours between them. If you cannot leave a 4-hour gap, skip the missed dose.
If you stop treatment with Cayston
Do not stop treatment with Cayston without consulting your doctor first.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you get a rash, see a doctor immediately,as this may mean that you are having an allergic reaction to Cayston.
Very common side effects (affect more than 1 in 10 people)
- Cough
- Nasal congestion
- Wheezing
- Sore throat
- Shortness of breath
- Fever. This may be more common in children than in adults.
Common side effects (affect between 1 and 10 in 100 people)
- Difficulty breathing
- Chest discomfort
- Nasal discharge
- Coughing up blood
- Rash
- Joint pain
- Lower results in lung function tests
Uncommon side effects (affect between 1 and 10 in 1,000 people)
- Joint swelling
The following side effects have been observed after the use of injectable aztreonam, but not after the administration of Cayston: swelling of the face, lips, tongue, or throat with difficulty swallowing or breathing, sweating, irritation, and peeling of the skin, itchy skin rash, redness, small red spots, and very rarely, blisters on the skin. All these signs may indicate an allergic reaction.
Tell your doctor if you get any of these side effects.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Cayston
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label of the vial, the solvent ampoule, and the packaging. The expiry date is the last day of the month stated.
Vial of powder and solvent ampoule:
Store in a refrigerator (between 2°C and 8°C). Unopened vials can also be stored outside the refrigerator, but at a temperature below 25°C for a maximum of 28 days.
Use this medicine immediately after preparation. If not used immediately, the prepared solution must be stored between 2°C and 8°C and used within a maximum of 8 hours. Do not prepare more than one dose at a time.
Do not use this medicine if you notice that the packaging has been tampered with.
Do not use this medicine if it has been stored outside the refrigerator for more than 28 days.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Cayston and Solvent
- The powder vial contains 75 mg of aztreonam (as lysine).
- The solvent ampoule contains water for injectable preparations and sodium chloride. The ampoule is printed in English only. The information that appears on the ampoule is presented below:

Appearance and Package Contents of the Product
Cayston is a white to off-white powder and solvent for nebulizer solution for inhalation.
Cayston is contained within a 2 ml amber glass vial with a gray rubber stopper and a removable aluminum seal with a blue closure cap.
The solvent (1 ml) is contained within a plastic ampoule.
Each 28-day package of Cayston contains 84 vials of Cayston lyophilized powder and 88 solvent ampoules. The four additional solvent ampoules are provided in case of spills.
This medication is available in:
- 28-day Cayston package
- Package containing a 28-day Cayston package and a handheld Altera nebulizer device
Only certain package sizes may be marketed.
Marketing Authorization Holder:
Gilead Sciences Ireland UC
Carrigtohill
County Cork, T45 DP77
Ireland
Manufacturer:
Gilead Sciences Ireland UC
IDA Business & Technology Park
Carrigtohill
County Cork
Ireland
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Belgium Gilead Sciences Belgium SPRL-BVBA Tel: + 32 (0) 2 401 35 50 | Lithuania Gilead Sciences Poland Sp. z o.o. Tel: + 48 22 262 8702 |
Greece Gilead Sciences Ireland UC Tel: + 353 (0) 1 686 1888 | Luxembourg Gilead Sciences Belgium SPRL-BVBA Tel: + 32 (0) 2 401 35 79 |
Czech Republic Gilead Sciences s.r.o. Tel: + 420 910 871 986 | Hungary Gilead Sciences Ireland UC Tel: + 353 (0) 1 686 1888 |
Denmark Gilead Sciences Sweden AB Tlf: + 46 (0) 8 5057 1849 | Malta Gilead Sciences Ireland UC Tel: + 353 (0) 1 686 1888 |
Germany Gilead Sciences GmbH Tel: + 49 (0) 89 899890-0 | Netherlands Gilead Sciences Netherlands B.V. Tel: + 31 (0) 20 718 36 98 |
Estonia Gilead Sciences Poland Sp. z o.o. Tel: + 48 22 262 8702 | Norway Gilead Sciences Sweden AB Tlf: + 46 (0) 8 5057 1849 |
Greece Gilead Sciences Ελλάς Μ.ΕΠΕ. Tel: +30 210 8930 100 | Austria Gilead Sciences GesmbH Tel: + 43 1 260 830 |
Spain Gilead Sciences, S.L. Tel: + 34 91 378 98 30 | Poland Gilead Sciences Poland Sp. z o.o. Tel: + 48 22 262 8702 |
France Gilead Sciences Tél: + 33 (0) 1 46 09 41 00 | Portugal Gilead Sciences, Lda. Tel: + 351 21 7928790 |
Croatia Gilead Sciences Ireland UC Tel: + 353 (0) 1 686 1888 | Romania Gilead Sciences Ireland UC Tel: + 353 (0) 1 686 1888 |
Ireland Gilead Sciences Ireland UC Tel: + 353 (0) 214 825 999 | Slovenia Gilead Sciences Ireland UC Tel: + 353 (0) 1 686 1888 |
Iceland Gilead Sciences Sweden AB Tel: + 46 (0) 8 5057 1849 | Slovakia Gilead Sciences Slovakia s.r.o. Tel: + 421 232 121 210 |
Italy Gilead Sciences S.r.l. Tel: + 39 02 439201 | Finland Gilead Sciences Sweden AB Tel: + 46 (0) 8 5057 1849 |
Cyprus Gilead Sciences Ελλάς Μ.ΕΠΕ. Tel: + 30 210 8930 100 | Sweden Gilead Sciences Sweden AB Tel: + 46 (0) 8 5057 1849 |
Latvia Gilead Sciences Poland Sp. z o.o. Tel: + 48 22 262 8702 | United Kingdom Gilead Sciences Ltd Tel: + 44 (0) 8000 113700 |
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medication is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CAYSTON 75 mg POWDER AND SOLVENT FOR SOLUTION FOR NEBULIZER INHALATIONDosage form: INJECTABLE, 1 g aztreonamActive substance: aztreonamManufacturer: Galenicum Derma S.L.U.Prescription requiredDosage form: INJECTABLE PERFUSION, 1.5/0.5 gActive substance: MonobactamsManufacturer: Pfizer Europe Ma EeigPrescription requiredDosage form: INJECTABLE, 1 gActive substance: meropenemManufacturer: Medochemie Iberia S.A.Prescription required
Online doctors for CAYSTON 75 mg POWDER AND SOLVENT FOR SOLUTION FOR NEBULIZER INHALATION
Discuss questions about CAYSTON 75 mg POWDER AND SOLVENT FOR SOLUTION FOR NEBULIZER INHALATION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















