BUCOSPRAY 15 mg/ml + 0.5 mg/ml ORAL SPRAY SOLUTION

How to use BUCOSPRAY 15 mg/ml + 0.5 mg/ml ORAL SPRAY SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
BUCOSPRAY 15 mg/ml + 0.5 mg/ml Oral Spray Solution
Chlorhexidine Gluconate / Benzocaine
Read the entire package leaflet carefully before starting to take this medication, as it contains important information for you.
Follow the administration instructions for the medication contained in this package leaflet or as indicated by your doctor or pharmacist.
- Keep this package leaflet, as you may need to read it again.
- If you need advice or more information, consult your pharmacist.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. See section 4.
- You should consult a doctor if your condition worsens or does not improve after 2 days of treatment.
Contents of the Package Leaflet:
- What is BUCOSPRAY and what is it used for
- What you need to know before taking BUCOSPRAY
- How to take BUCOSPRAY
- Possible side effects
- Storage of BUCOSPRAY
- Package Contents and Additional Information
1. What is Bucospray and what is it used for
This medication combines the antiseptic action of chlorhexidine with the local anesthetic action of benzocaine. While the antiseptic provides disinfection in the buco-pharyngeal area, the local anesthetic relieves pain and calms discomfort.
Indicated for adults and children from 6 years old for the symptomatic relief of mild mouth and throat infections that occur without fever and with pain, such as: throat irritation, hoarseness, small wounds, and canker sores.
You should consult a doctor if your condition worsens or does not improve after 2 days of treatment.
2. What you need to know before taking Bucospray
Do not use BUCOSPRAY
- If you are allergic to chlorhexidine, benzocaine, or any other component of this medication (listed in section 6).
- If you do not tolerate other local anesthetics like para-aminobenzoic acid (PABA), parabens, or para-phenylenediamine (a hair dye component).
Warnings and Precautions
Consult your doctor or pharmacist before starting to use BUCOSPRAY
- If you have any serious or extensive injury in the mouth.
- If you have periodontitis (gum disease), as chlorhexidine may increase supragingival calculus.
- Do not take higher doses than recommended in section 3 (How to take BUCOSPRAY).
- If you have asthma
- Do not use before eating or drinking.
- If you have dental fillings and the surface of the filling or its edges are rough, chlorhexidine can cause permanent discoloration.
- It is recommended to maintain good oral hygiene to reduce plaque accumulation and possible tooth discoloration that may be caused by chlorhexidine.
Interference with analytical tests
If you are going to undergo any diagnostic test (including blood tests, urine tests, skin tests using allergens, etc.), inform your doctor that you are using this medication, as it may alter the results.
This medication may interact with pancreatic function tests using bentiromide. Do not take this medication at least 3 days before the test and inform your doctor.
Children
This medication should not be used in children under 6 years old.
Between 6 and 12 years old, it can only be used under adult supervision.
Using BUCOSPRAY with other medications
Inform your doctor or pharmacist if you are taking, have recently taken, or may need to take any other medication.
Although not described in the recommended usage conditions, do not use with other mouth or throat medications without consulting your doctor or pharmacist.
This is especially important in the case of:
- Other mouth or throat antiseptics.
- Cholinesterase inhibitor medications (medications for Alzheimer's disease).
- Sulfonamides (used for infections).
Anionic compounds and suspending agents, common components of toothpastes, reduce the effectiveness of chlorhexidine, so the mouth should be rinsed well after using toothpaste.
Pregnancy and Breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
Driving and Using Machines
Using this medication does not affect your ability to drive or operate machines.
Use in People Over 65
Older people and debilitated patients may be more sensitive to the adverse effects of benzocaine.
3. How to Take Bucospray
Follow the administration instructions for the medication contained in this package leaflet or as indicated by your doctor or pharmacist. In case of doubt, ask your doctor or pharmacist.
The recommended dose is:
Adults and adolescents over 12 years old:
2 sprays every 2 or 3 hours, increasing the time between applications as discomfort decreases. In no case should more than 16 sprays be used per day.
Children from 6 to 12 years old:
1 spray every 2 or 3 hours. In no case should more than 8 sprays be used per day.
How to use
This medication is for buco-pharyngeal use
The medication has a local action, so it should be avoided swallowing or aspirating the medication.
Do not use this medication before meals or before drinking.
Before using the bottle for the first time, it is convenient to perform 2 or 3 sprays in the air to prime the dosing valve.
Always use the smallest effective dose.
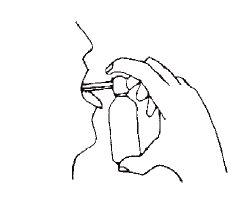
| To correctly apply BUCOSPRAY, follow the instructions in the drawing. Place the depressor-nozzle of the dosing valve in the open mouth, gently pressing the tongue, and then press down 1 or 2 times. |
You should consult a doctor if your condition worsens or does not improve after 2 days of treatment.
If you notice that after 2 days of starting treatment you have a fever, headache, nausea, or vomiting, you should consult your doctor as soon as possible.
If you use more BUCOSPRAY than you should
If you use more BUCOSPRAY than you should, you may notice slurred speech, numbness, staggering gait, blurred or double vision, dizziness, excitement, or convulsions, ringing in the ears, increased sweating. You may also experience a decrease in blood pressure.
In case of overdose or accidental ingestion, consult your doctor, pharmacist, or call the Toxicology Information Service, phone 915 62 04 20, indicating the medication and the amount taken.
4. Possible Side Effects
Like all medications, this medication can cause side effects, although not everyone experiences them.
Tooth discoloration may appear, especially in people with plaque accumulation on their teeth. This alteration of tooth color is not permanent and can be removed by a dental cleaning. The color of dental fillings can also be altered, in which case this discoloration can be permanent.
Alteration in taste perception may occur.
In some cases, BUCOSPRAY may cause irritation in the mouth or irritation of the tip of the tongue, which are usually transient.
Reporting Side Effects
If you experience any type of side effect, consult your doctor or pharmacist, even if it is a possible side effect not listed in this package leaflet. You can also report them directly through the Spanish Medication Surveillance System website: www.notificaram.es.
By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Bucospray
Keep this medication out of sight and reach of children.
Do not store at a temperature above 25°C.
Do not use this medication after the expiration date shown on the packaging, after the abbreviation CAD. The expiration date is the last day of the month indicated.
Medications should not be disposed of through wastewater or household waste. Deposit the packaging and medications you no longer need at the SIGRE point in the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medications you no longer need. This will help protect the environment.
6. Package Contents and Additional Information
Composition of BUCOSPRAY
- The active ingredients are chlorhexidine gluconate and benzocaine. Each ml of solution contains 0.5 mg of chlorhexidine gluconate (each spray of 0.05 ml contains 0.025 mg) and 15 mg of benzocaine (each spray of 0.05 ml contains 0.75 mg).
- The other components (excipients) are: sodium saccharin, 96% ethanol, glycerol, ammonium glycyrrhizate, and strawberry flavor.
Appearance of the Product and Package Contents
BUCOSPRAY is presented in a spray bottle containing 25 ml of solution, provided with a dosing valve.
Marketing Authorization Holder and Manufacturer
Teofarma S.r.l.
Via F.lli Cervi 8
27010 VALLE SALIMBENE (Pavia), ITALY
Date of the Last Revision of this Package Leaflet: July 2014
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BUCOSPRAY 15 mg/ml + 0.5 mg/ml ORAL SPRAY SOLUTIONDosage form: TOPICAL ORAL PRODUCT, 10 mg/mlActive substance: povidone-iodineManufacturer: Cooper Consumer Health B.V.Prescription not requiredDosage form: GEL/PASTE/ORAL LIQUID, 10% povidone iodineActive substance: povidone-iodineManufacturer: Cooper Consumer Health B.V.Prescription not requiredDosage form: BUCCAL TABLET/LOZENGE, 2mg lidocaine/0.6mg amylmetacresol/1.2mg diclofenac alcoholActive substance: variousManufacturer: Laboratorios Cinfa S.A.Prescription not required
Online doctors for BUCOSPRAY 15 mg/ml + 0.5 mg/ml ORAL SPRAY SOLUTION
Discuss questions about BUCOSPRAY 15 mg/ml + 0.5 mg/ml ORAL SPRAY SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions