
BRIPIO 2 mg/ml EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERS

How to use BRIPIO 2 mg/ml EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
BRIPIO2 mg/ml eye drops, solution in single-dose container
Brimonidine tartrate
Read all of this leaflet carefully before you start using this medicine.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the package leaflet
- What BRIPIO is and what it is used for
- What you need to know before you use BRIPIO
- How to use BRIPIO
- Possible side effects
- Storage of BRIPIO
- Contents of the pack and other information
1. What BRIPIO is and what it is used for
BRIPIO is used to lower high pressure in the eye. The active substance in BRIPIO is brimonidine tartrate, which belongs to a group of medicines called alpha-2 adrenergic receptor agonists and works by reducing pressure in the eyeball.
It can be used alone, when beta-blocker eye drops are contraindicated, or with other eye drops, when the medicine on its own is not enough to lower increased pressure in the eye in the treatment of open-angle glaucoma or ocular hypertension.
2. What you need to know before you use BRIPIO
Do not useBRIPIO
- If you are allergic to brimonidine tartrate or any of the other ingredients of BRIPIO (listed in section 6).
- If you are taking monoamine oxidase inhibitors (MAOIs) or other antidepressants. You should tell your doctor if you are taking any antidepressant medication.
- If you are breast-feeding.
- In children/babies (up to 2 years of age).
Be cautious withBRIPIO
Before starting treatment with BRIPIO, tell your doctor:
- If you have or have had depression, decreased mental ability, reduced blood flow to the brain, heart problems, a disorder of blood supply to the limbs, or a blood pressure disorder.
- If you have or have had kidney or liver problems.
Children and adolescents
BRIPIO is not recommended for use in children between 2 and 12 years old.
BRIPIO should not be used in adolescents from 12 to 17 years old, as clinical studies have not been conducted in this age group.
Use of other medicines
Tell your doctor or pharmacist if you are taking/using, have recently taken/used, or might take/use any other medicines.
Tell your doctor if you are using any of the following medicines:
- Pain relievers, sedatives, opioids, barbiturates, or if you are regularly consuming alcohol.
- Anesthetics.
- Medicines for heart conditions or to lower blood pressure.
- Medicines that may affect metabolism, such as chlorpromazine, methylphenidate, and reserpine.
- Medicines that act on the same receptor as BRIPIO, such as isoprenaline and prazosin.
- MAOIs and other antidepressants.
- Medicines for any condition, including those not related to your eye condition.
These may affect your treatment with BRIPIO.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
BRIPIO should not be used during breast-feeding. Do not use BRIPIO if you are pregnant unless your doctor considers it necessary.
Driving and using machines
- BRIPIO may cause blurred or abnormal vision. This effect may be increased in the dark or with reduced lighting.
- BRIPIO may also cause drowsiness or fatigue in some patients.
If you experience any of these symptoms, do not drive or use machines until the symptoms have resolved.
3. How to use BRIPIO
Follow the instructions for administration given by your doctor. Ask your doctor or pharmacist if you have any doubts.
Adults
The normal dose is one drop twice a day in the affected eye(s), approximately 12 hours apart. Do not change the dose or stop using BRIPIO without talking to your doctor.
Children under 12 years
BRIPIO should not be used in children under 2 years of age.
BRIPIO is not recommended for use in children between 2 and 12 years old.
Instructions for use
- Wash your hands.
- Open the aluminum bag and remove the strip of single-dose containers.
- Separate one single-dose container from the strip (Figure 1).
- Put the rest of the single-dose containers back in the aluminum bag and close it by folding the edge. Put the bag back in the box.
- Open the single-dose container by twisting the cap until it comes off. Do not touch the tip of the container once opened (Figure 2).
- Tilt your head back (Figure 3).
- Gently pull down the lower eyelid with your finger, hold the single-dose container with your other hand with the tip facing down. Squeeze the container to release one drop into the affected eye (Figure 4).
- Keep the affected eye closed, press your finger against the inner corner of the closed eye for 1 minute. This will prevent the drop from entering the tear duct and going down the throat, so most of the drop will stay in the eye (Figure 5). If necessary, repeat steps 6 to 8 with the other eye.
- Discard the single-dose container after use.
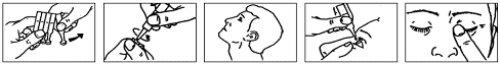
Figure 1 Figure 2 Figure 3 Figure 4 Figure 5
If the drop falls outside the eye, try again.
If you use BRIPIO with another eye drop, wait 5-15 minutes before applying the second eye drop.
If you use moreBRIPIOthan you should
Adults
In adults who applied more drops than prescribed, the adverse effects reported were those already known with brimonidine.
Adults who accidentally ingested brimonidine experienced a decrease in blood pressure, which in some patients was followed by an increase in blood pressure.
Children
Severe adverse effects were reported in children who had accidentally ingested brimonidine. The signs included drowsiness, hypotonia, low body temperature, paleness, and breathing difficulties. If this happens, contact your doctor immediately.
Adults and children
If you have accidentally ingested BRIPIO or used more BRIPIO than you should, please contact your doctor immediately.
Go to your doctor or pharmacist immediately. Take the medicine package with you. You can also call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount taken.
If you forget to useBRIPIO
If you forget to apply a dose, apply it as soon as you remember. However, if it is almost time for the next dose, you should omit the missed dose and then follow your regular schedule.
Do not use a double dose to make up for missed doses.
If you stop usingBRIPIO
BRIPIO should be used every day to be effective. Do not stop using BRIPIO until your doctor tells you to.
If you have any doubts about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects may occur with both brimonidine in multidose containers, with preservatives, and brimonidine in single-dose containers, without preservatives.
Affecting the eye
Very common (may affect more than 1 in 10 people)
- Eye irritation (redness of the eye, burning, stinging, feeling of a foreign body in the eye, or itching, follicles or white spots on the transparent layer covering the surface of the eye)
- Blurred vision
- An allergic reaction in the eyes
Common (may affect up to 1 in 10 people)
- Local irritation (inflammation and swelling of the eyelid, swelling of the transparent layer covering the surface of the eye, sticky eyes, pain, and tearing)
- Sensitivity to light
- Erosion on the surface of the eye and discoloration
- Dry eye
- Whitening of the transparent layer covering the surface of the eye
- Abnormal vision
- Inflammation of the transparent layer covering the surface of the eye
Very rare (may affect up to 1 in 10,000 people)
- Inflammation in the eye
- Reduced pupil size
Frequency not known (cannot be estimated from the available data)
- Itching of the eyelids
- Inflammation of the iris, the colored part of the eye, and the ciliary bodies, muscles, and tissue involved in focusing vision (Iridocyclitis). This disease is also known as "anterior uveitis"
Affecting the rest of the body
Very common (may affect more than 1 in 10 people)
- Headache
- Dry mouth
- Fatigue/drowsiness
Common (may affect up to 1 in 10 people)
- Dizziness
- Cold symptoms
- Symptoms affecting the stomach and digestion
- Altered taste
- General weakness
Uncommon (may affect up to 1 in 100 people)
- Depression
- Palpitations or changes in heart rate
- Dry nose
- General allergic reactions
Rare (may affect up to 1 in 1,000 people)
- Breathing difficulties
Very rare (may affect up to 1 in 10,000 people)
- Insomnia
- Fainting
- High blood pressure
- Low blood pressure
Frequency not known (cannot be estimated from the available data)
- Skin reactions including redness, inflammation of the face, itching, rash, and dilation of blood vessels.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Medicines and Healthcare Products Agency (AEMPS) website: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of BRIPIO
Keep this medicine out of the sight and reach of children.
Store the single-dose containers in the aluminum bag to protect them from light.
Once the aluminum bag is opened, do not use after 3 months.
BRIPIO does not contain preservatives. Once the single-dose container is opened, the contents should be used immediately. You should discard the rest of the medicine in the single-dose container after application.
Do not use this medicine after the expiry date which is stated on the carton, the aluminum bag, and on the single-dose container after EXP. The expiry date is the last day of the month shown.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
Composition ofBRIPIO2 mg/ml eye drops, solution in single-dose container
- The active substance is brimonidine tartrate. One ml of solution contains 2.0 mg of brimonidine tartrate, equivalent to 1.3 mg of brimonidine. One drop contains 0.06-0.07 mg of brimonidine tartrate.
- The other ingredients are poly(vinyl alcohol), sodium chloride, sodium citrate, citric acid monohydrate, water for injections, and sodium hydroxide or hydrochloric acid for pH adjustment.
Appearance and packaging of the product
BRIPIO 2 mg/ml eye drops, solution in single-dose container is a clear, slightly yellowish-green solution. The content of one single-dose container is 0.35 ml of solution. Each aluminum bag contains two strips of 5 single-dose containers.
BRIPIO 2 mg/ml eye drops, solution in single-dose container is available in 10, 20, 30, 50, 60, 100, or 120 single-dose containers with 0.35 ml of solution each.
Not all packs may be marketed.
Marketing authorization holder and manufacturer
Pharma Stulln GmbH
Werksstrasse 3
92551 Stulln
Germany
You can obtain further information on this medicine from the local representative of the marketing authorization holder:
BRILL PHARMA, S.L.
C/ Munner, 8
08022 Barcelona
Spain
This medicine is authorized in the Member States of the European Economic Area under the following names:
Member State | Medicine name | |
Austria |
| |
France |
| |
Germany |
| |
Netherlands |
| |
Spain |
| |
Greece | Brimofree 2 mg/ml Οφθαλμικ?ς σταγ?νες, δι?λυμα σε περι?κτη μ?ας δ?σης |
Date of last revision of this leaflet: July 2023
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Healthcare Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price30.69 EUR
- Availability in pharmacies
Supply issue reported
Data from the Spanish Agency of Medicines (AEMPS) indicates a supply issue affecting this medicine.<br><br>Availability may be limited in some pharmacies.<br><br>For updates or alternatives, consult your pharmacist. - Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BRIPIO 2 mg/ml EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERSDosage form: EYE DROP, 2 mg/mlActive substance: brimonidineManufacturer: Tiedra Farmaceutica S.L.Prescription requiredDosage form: EYEDROP, 2 mg/ml of brimonidine tartrateActive substance: brimonidineManufacturer: Abbvie Spain, S.L.U.Prescription requiredDosage form: EYEDROP, 2 MG/MLActive substance: brimonidineManufacturer: Viatris LimitedPrescription required
Online doctors for BRIPIO 2 mg/ml EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERS
Discuss questions about BRIPIO 2 mg/ml EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















