
BRIMVERA 2 mg/ml EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERS

How to use BRIMVERA 2 mg/ml EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Brimvera 2mg/ml eye drops, solution in single-dose container
Brimonidine tartrate
Read this package leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this package leaflet, you may need to read it again. If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. See section 4.
Contents of the package leaflet
- What is Brimvera and what is it used for
- What you need to know before you use Brimvera
- How to use Brimvera
- Possible side effects
- Storage of Brimvera
- Contents of the pack and further information
1. What is Brimvera and what is it used for
Brimvera is used to reduce intraocular pressure.
It can be used alone, when beta-blocker eye drops are contraindicated, or with other eye drops, when the medicine alone is not sufficient to reduce an increase in intraocular pressure in the treatment of open-angle glaucoma or ocular hypertension.
The active substance of Brimvera is brimonidine tartrate, which acts by reducing the pressure inside the eye.
2. What you need to know before you use Brimvera
Do not use Brimvera:
- If you are allergic to brimonidine tartrate or any of the other ingredients of this medicine (listed in section 6).
- If you are taking monoamine oxidase inhibitors (MAOIs) or certain antidepressants. You should inform your doctor if you are taking any antidepressant medication.
- If you are breast-feeding.
- In children from birth to 2 years of age.
Warnings and precautions
Before starting treatment with Brimvera, inform your doctor:
- If you suffer or have suffered from depression, decreased mental capacity, reduced blood flow to the brain, heart problems, altered blood supply to the limbs, or a blood pressure disorder.
- If you have or have had kidney or liver problems in the past.
- In the event that it is being administered to a child between 2 and 12 years of age, since the use of Brimvera is not recommended in this age group.
Children and adolescents
Clinical studies have not been conducted in adolescents (12 to 17 years).
The use of Brimvera is not recommended in children under 12 years of age and is contraindicated in newborns and children under 2 years of age.
Other medicines and Brimvera
Tell your doctor or pharmacist if you are taking or have recently taken or might take any other medicines.
Tell your doctor if you are taking any of the following medicines:
- for pain, sedatives, opioids, barbiturates, or if you regularly consume alcohol.
- anesthetics.
- medicines to treat a heart condition or to lower blood pressure.
- medicines that can affect metabolism, such as chlorpromazine, methylphenidate, and reserpine.
- medicines that act on the same receptor as Brimvera, such as isoprenaline and prazosin.
- monoamine oxidase inhibitors (MAOIs) and other antidepressants.
- medicines for any condition, including those not related to your eye condition.
- or if the dose of any of your current medicines changes.
These may affect your treatment with Brimvera.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Brimvera should not be used during breast-feeding.
Driving and using machines
- Brimvera may cause blurred or abnormal vision. This effect can be exacerbated at night or with reduced lighting.
- Brimvera can also cause drowsiness or fatigue in some patients.
If you experience any of these symptoms, do not drive or use machines until the symptoms have resolved.
3. How to use Brimvera
Follow the administration instructions indicated by your doctor exactly. Consult your doctor or pharmacist if you have any doubts.
Use in adults
The recommended dose is one drop, twice a day, in the affected eye(s), approximately 12 hours apart.
Use in children under 12 years of age
Brimvera should not be used in children under 2 years of age.
The use of Brimvera is not recommended in children between 2 and 12 years of age.
Instructions for use
- Wash your hands.
- Open the aluminum pouch and remove the block of single-dose containers.
- Separate a single-dose container from the strip (Fig. 1).
- Place the remaining single-dose containers in the pouch and close it by folding the edge. Place the pouch in the carton box.
- Open the single-dose container by twisting the tip. Do not touch the tip after opening the container (Fig. 2).
- Tilt your head back (Fig. 3).
- Pull down the lower eyelid with your finger and hold the single-dose container in your other hand. Squeeze the container to release one drop into the eye (Fig. 4).
- Close your eyes and press the inner corner of the eye with the tip of a finger for about 1 minute. This will prevent the drop from passing through the tear duct into the throat, and most of the drop will remain in the eye (Fig. 5). If necessary, repeat steps 6 to 8 with your other eye.
- After use, discard the single-dose container.
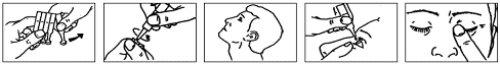
Fig. 1 Fig. 2 Fig. 3 Fig. 4 Fig. 5
If you use Brimvera with another eye drop, wait 5-15 minutes before applying the second eye drop.
If you use more Brimvera than you should
Adults
In adults who applied more drops than prescribed, the adverse effects reported were those already known for brimonidine.
Adults who accidentally ingested brimonidine eye drops experienced a decrease in blood pressure, which in some patients was followed by an increase in blood pressure.
Children
Serious adverse effects were reported in children who had accidentally ingested brimonidine eye drops. The signs included drowsiness, feeling of muscle weakness or fatigue, low body temperature, paleness, and breathing difficulties. If this happens, contact your doctor immediately.
Adults and children
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately, or call the Toxicological Information Service, phone 91 562 04 20.
If you forget to use Brimvera
If you forget to administer a dose, apply it as soon as you remember. However, if it is almost time for your next dose, you should omit the forgotten dose and then follow your regular schedule.
Do not use a double dose to make up for forgotten doses.
If you stop using Brimvera
To be effective, Brimvera must be used every day. Do not stop using Brimvera until your doctor tells you to.
If you have any doubts about the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects have been observed with brimonidine eye drops with preservatives in multidose containers and may also occur when using brimonidine eye drops without preservatives in single-dose containers:
Affecting the eye
Very common (may affect more than 1 in 10 patients):
- Eye irritation (redness of the eye, burning, stinging, sensation of a foreign body in the eye, itching, follicles or white spots on the transparent layer covering the surface of the eye)
- Blurred vision
- Allergic reaction in the eye
Common (may affect up to 1 in 10 patients):
- Local irritation (inflammation and swelling of the eyelid, swelling of the transparent layer covering the surface of the eye, sticky eyes, pain, and tearing)
- Sensitivity to light
- Erosion on the surface of the eye and discoloration
- Dry eye
- Whitening of the transparent layer covering the surface of the eye
- Abnormal vision
- Inflammation of the transparent layer covering the surface of the eye
Very rare (may affect up to 1 in 10,000 patients):
- Inflammation in the eye
- Reduced pupil size
Side effects with unknown frequency (frequency cannot be estimated from available data):
- Itching of the eyelids
- Inflammation of the iris, i.e., the colored part of the eye, and the ciliary body, i.e., muscles and tissue involved in focusing the eye (iridocyclitis). This condition is also called "anterior uveitis"
Affecting the body
Very common (may affect more than 1 in 10 patients):
- Headache
- Dry mouth
- Fatigue/drowsiness
Common (may affect up to 1 in 10 patients):
- Dizziness
- Cold symptoms
- Symptoms affecting the stomach and digestion
- Altered taste
- General weakness
Uncommon (may affect up to 1 in 100 patients):
- Depression
- Palpitations or changes in heart rate
- Nasal dryness
- General allergic reactions
Rare (may affect up to 1 in 1,000 patients):
- Respiratory failure
Very rare (may affect up to 1 in 10,000 patients):
- Insomnia
- Fainting
- High blood pressure
- Low blood pressure
Side effects with unknown frequency (frequency cannot be estimated from available data):
- Skin reactions including redness, facial inflammation, itching, rash, and dilation of blood vessels
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. You can also report side effects directly through the Spanish Medicines Surveillance System for Human Use, Website: www.notificaram.es.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Brimvera
Keep this medicine out of the sight and reach of children.
Store the single-dose containers in the aluminum pouch to protect them from light.
Do not use after 3 months from opening the aluminum pouch.
Brimvera does not contain preservatives. Once opened, the contents of a single-dose container should be used immediately. You should discard the remaining solution in the container after application.
Do not use this medicine after the expiry date which is stated on the carton, pouch, and single-dose container after EXP. The expiry date is the last day of the month shown.
Medicines should not be disposed of via wastewater or household waste. Place the containers and medicines you no longer need in the SIGRE collection point at the pharmacy. If in doubt, ask your pharmacist how to dispose of the containers and medicines you no longer need. This will help protect the environment.
6. Contents of the pack and further information
Composition of Brimvera
- The active substance is brimonidine tartrate. 1 ml of solution contains 2.0 mg of brimonidine tartrate, equivalent to 1.3 mg of brimonidine. One drop contains 0.06-0.07 mg of brimonidine tartrate.
- The other ingredients are poly(vinyl alcohol), sodium chloride, sodium citrate, citric acid monohydrate, water for injections, and hydrochloric acid or sodium hydroxide (for pH adjustment).
Appearance and contents of the pack
Brimvera is a clear, slightly yellow-green solution. A single-dose container contains 0.35 ml of solution. An aluminum pouch contains two strips of 5 single-dose containers each.
Brimvera is marketed in cartons containing 30, 60, or 120 single-dose containers with 0.35 ml of solution each.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
Esteve Pharmaceuticals, S.A.
Passeig de la Zona Franca, 109
08038 Barcelona
Manufacturer
Pharma Stulln GmbH
Werksstraße 3
92551 Stulln
Germany
This medicine is authorized in the Member States of the European Economic Area under the following names:
Austria Brimonidin sine OmniVision
France Brimonidine OmniVision
Italy Brimofree
Spain Brimvera
United Kingdom Brimonidine OmniVision
This package leaflet was approvedJuly 2023
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price15.35 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BRIMVERA 2 mg/ml EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERSDosage form: EYE DROP, 2 mg/mlActive substance: brimonidineManufacturer: Tiedra Farmaceutica S.L.Prescription requiredDosage form: EYEDROP, 2 mg/ml of brimonidine tartrateActive substance: brimonidineManufacturer: Abbvie Spain, S.L.U.Prescription requiredDosage form: EYEDROP, 2 MG/MLActive substance: brimonidineManufacturer: Viatris LimitedPrescription required
Online doctors for BRIMVERA 2 mg/ml EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERS
Discuss questions about BRIMVERA 2 mg/ml EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















