ADZYNMA 1500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION

How to use ADZYNMA 1500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
ADZYNMA 500UI powder and solvent for solution for injection
ADZYNMA 1500UI powder and solvent for solution for injection
rADAMTS13
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- 1. What ADZYNMA is and what it is used for
- What you need to know before you use ADZYNMA
- How to use ADZYNMA
- Possible side effects
- Storage of ADZYNMA
- Contents of the pack and other information
- Instructions for use
1. What ADZYNMA is and what it is used for
ADZYNMA contains the active substance rADAMTS13, a synthetic copy of the natural enzyme (protein) ADAMTS13. People with congenital thrombotic thrombocytopenic purpura (TTP) lack this enzyme.
Congenital TTP is a very rare inherited blood disorder that causes blood clots to form in small blood vessels. These clots can block the flow of blood and oxygen to organs, resulting in a lower than normal number of platelets (components of blood that help it clot) in the blood.
Congenital TTP is caused by a lack of the ADAMTS13 enzyme in the blood. ADAMTS13 helps prevent blood clots from forming by breaking down large molecules called von Willebrand factor (VWF). When VWF molecules are too large, they can cause dangerous blood clots. ADZYNMA is used to replace ADAMTS13 levels, which helps break down these large molecules into smaller ones. This reduces the likelihood of blood clots forming and can prevent low platelet counts in people with TTP.
2. What you need to know before you use ADZYNMA
Do not use ADZYNMA
- If you have had severe or life-threatening allergic reactions to rADAMTS13 or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start using ADZYNMA.
Allergic reactions
There is a risk of having an allergic reaction with ADZYNMA. Your doctor should tell you about the immediate signs of severe allergic reactions, such as:
- rapid heartbeat
- chest tightness
- wheezing and/or sudden difficulty breathing
- low blood pressure
- hives, rash, and itching of the skin
- runny or stuffy nose
- red eyes
- sneezing
- rapid swelling under the skin in areas such as the face, throat, arms, and legs
- fatigue
- nausea (feeling sick)
- vomiting
- feeling of numbness, tingling, or muscle pain
- restlessness
- anaphylaxis (a severe allergic reaction that can cause difficulty swallowing and/or breathing and redness or swelling of the face and/or hands).
If you have any of these symptoms, your doctor will decide whether treatment with ADZYNMA should be stopped and will provide you with the necessary medication to treat the allergic reaction. Severe symptoms, including difficulty breathing and dizziness, require urgent treatment.
Inhibitors
Neutralizing antibodies (called inhibitors) may develop in some patients who receive ADZYNMA. These inhibitors could potentially cause the treatment to stop working properly. Tell your doctor if you think the medicine is not working for you.
Other medicines and ADZYNMA
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
You should not use ADZYNMA during pregnancy unless your doctor specifically recommends it. You and your doctor will need to decide if you can use ADZYNMA during breastfeeding.
Driving and using machines
The ability to drive and use machines may be small. After using ADZYNMA, dizziness and drowsiness may occur.
Keeping a record
In order to improve the traceability of biological medicines, the name and batch number of the medicine should be clearly recorded.
ADZYNMA contains sodium
This medicine contains less than 23 mg of sodium (1 mmol) per vial; this is essentially "sodium-free".
ADZYNMA contains polysorbate 80
This medicine contains 2.7 mg of polysorbate 80 in each vial of ADZYNMA 500 UI or 1,500 UI, equivalent to up to 0.216 mg/kg. Polysorbates can cause allergic reactions. Tell your doctor if you have any known allergies.
3. How to use ADZYNMA
Treatment with ADZYNMA will be given under the supervision of a doctor with experience in treating patients with blood disorders.
ADZYNMA is given by injection into a vein. It is provided to your doctor as a powder that is dissolved (reconstituted) with the solvent (a liquid in which the powder can be dissolved) supplied before administration.
The dose is calculated based on your body weight.
Administration of the medicine at home
Your doctor may consider using ADZYNMA at home if you tolerate injections well. When you are able to inject ADZYNMA (or have it administered by a caregiver) after receiving the relevant training from the doctor or nurse responsible for your treatment, your doctor will continue to monitor your response to treatment. If you notice any side effects when using the medicine at home, stop the injection immediately and seek medical attention.
Recommended dose
Preventive enzyme replacement therapy
The usual dose is 40 UI per kilogram of body weight, given every two weeks.
Your doctor may change the frequency to once a week if administration of ADZYNMA every two weeks does not work for you.
On-demand enzyme replacement therapy for acute TTP episodes
If you develop an acute episode of thrombotic thrombocytopenic purpura (TTP), the recommended dose of ADZYNMA is as follows:
- 40 UI/kg of body weight on day 1.
- 20 UI/kg of body weight on day 2.
- 15 UI/kg of body weight once daily, starting on day 3 and until two days after the acute TTP episode has resolved.
If you use more ADZYNMA than you should
Using too much of this medicine can cause bleeding.
If you forget to use ADZYNMA
If you have missed an injection of ADZYNMA, tell your doctor as soon as possible. Do not take a double dose to make up for forgotten doses.
If you stop using ADZYNMA
If you want to stop treatment with ADZYNMA, talk to your doctor. If you stop treatment, the symptoms of the disease may worsen.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects have been reported with ADZYNMA:
Very common(may affect more than 1 in 10 people)
- nasal and throat infection
- headache
- feeling dizzy
- migraine
- diarrhea
- nausea
Common(may affect up to 1 in 10 people)
- high platelet count in the blood (thrombocytosis)
- drowsiness
- constipation
- bloating (abdominal distension)
- weakness (asthenia)
- feeling hot
- abnormal ADAMTS13 activity
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of ADZYNMA
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and carton after EXP. The expiry date is the last day of the month stated.
Unopened vials
Store in a refrigerator (2°C - 8°C).
Do not freeze.
Store in the original package to protect from light.
Unopened ADZYNMA powder vials may be stored at room temperature (up to 30°C) for a period of up to 6 months, but not beyond the expiry date. Do not put ADZYNMA back in the refrigerator after it has been stored at room temperature. Record the date you removed ADZYNMA from the refrigerator on the carton.
After reconstitution
Discard the reconstituted medicine that is not used within 3 hours.
Do not use this medicine if you notice it is not clear and colorless.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Composition of ADZYNMA
- The active substance, ADAMTS13, is a recombinant human "disintegrin A and metalloprotease with thrombospondin motifs 13" that has been purified.
The nominal content of each vial of powder is 500 or 1,500 IU of rADAMTS13 activity.
- The vial of solvent contains 5 ml of water for injectable preparations.
- The other excipients are sodium chloride, calcium chloride dihydrate, L-histidine, mannitol, sucrose, and polysorbate 80 (E433). See section 2 "ADZYNMA contains sodium" and "ADZYNMA contains polysorbate 80".
Appearance of the Product and Container Contents
ADZYNMA is supplied as a powder and solvent for injectable solution. The powder is a white lyophilized powder. The solvent is clear and colorless.
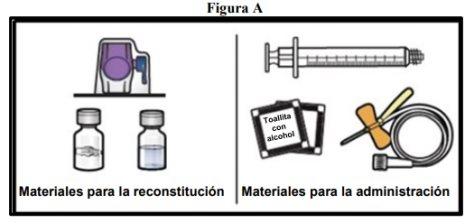
Each container contains a vial of powder, a vial of solvent, a reconstitution device (BAXJECT II Hi-Flow), a disposable syringe, an infusion set, and two alcohol swabs.
Marketing Authorization Holder and Manufacturer
Takeda Manufacturing Austria AG
Industriestrasse 67
1221 Vienna
Austria
You can request more information about this medicinal product by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien Takeda Belgium NV Tel: +32 2 464 06 11 | Lietuva Takeda, UAB Tel: +370 521 09 070 |
| Luxembourg/Luxemburg Takeda Belgium NV Tel: +32 2 464 06 11 |
Ceská republika Takeda Pharmaceuticals Czech Republic s.r.o. Tel: +420 234 722 722 | Magyarország Takeda Pharma Kft. Tel.: +36 1 270 7030 |
Danmark Takeda Pharma A/S Tlf: +45 46 77 10 10 | Malta Takeda HELLAS S.A. Tel: +30 210 6387800 |
Deutschland Takeda GmbH Tel: +49 (0)800 825 3325 | Nederland Takeda Nederland B.V. Tel: +31 20 203 5492 |
Eesti Takeda Pharma OÜ Tel: +372 6177 669 | Norge Takeda AS Tlf: +47 800 800 30 |
| Österreich Takeda Pharma Ges.m.b.H. Tel: +43 (0) 800-20 80 50 |
España Takeda Farmacéutica España, S.A. Tel: +34 917 90 42 22 | Polska Takeda Pharma Sp. z o.o. Tel.: +48223062447 |
France Takeda France SAS Tél: + 33 1 40 67 33 00 | Portugal Takeda Farmacêuticos Portugal, Lda. Tel: + 351 21 120 1457 |
Hrvatska Takeda Pharmaceuticals Croatia d.o.o. Tel: +385 1 377 88 96 | România Takeda Pharmaceuticals SRL Tel: +40 21 335 03 91 |
Ireland Takeda Products Ireland Ltd Tel: 1800 937 970 | Slovenija Takeda Pharmaceuticals farmacevtska družba d.o.o. Tel: + 386 (0) 59 082 480 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika Takeda Pharmaceuticals Slovakia s.r.o. el: +421 (2) 20 602 600 |
Italia Takeda Italia S.p.A. Tel: +39 06 502601 | Suomi/Finland Teva Finland Oy Puh/Tel: +358 20 180 5900 |
| Sverige Takeda Pharma AB Tel: 020 795 079 |
Latvija Takeda Latvia SIA Tel: +371 67840082 |
Date of Last Revision of this Leaflet: 08/2024.
This medicinal product has been authorized under "exceptional circumstances". This means that due to the rarity of this disease, it has not been possible to obtain complete information on this medicinal product.
The European Medicines Agency will review any new information on this medicinal product that may become available annually, and this leaflet will be updated as necessary.
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: https://www.ema.europa.eu. There are also links to other websites on rare diseases and orphan medicines.
- Instructions for Use
These instructions contain information on how to reconstitute and infuse ADZYNMA.
These instructions for use are intended for healthcare professionals and patients/caregivers who administer ADZYNMA at home after receiving proper training from a healthcare professional.
Treatment with ADZYNMA should be prescribed and supervised by a healthcare professional with experience in the treatment of patients with blood disorders.
Important:
- For intravenous injection only after reconstitution.
- Use aseptic technique throughout the procedure.
- Check the expiration date of the product before using it.
- Do notuse ADZYNMA if the expiration date has passed.
- If a patient needs more than one vial of ADZYNMA per injection, reconstitute each vial according to the detailed instructions provided in "Reconstitution".
- Inspect the reconstituted ADZYNMA solution for particles or color changes before administration. The solution should be clear and colorless.
- Do notadminister it if you observe particles or color changes.
- If stored at room temperature, use ADZYNMA before 3 hourshave passed since reconstitution.
- Do notadminister ADZYNMA in the same tube or container and simultaneously with other infusion medications.
Reconstitution
- Prepare a clean and flat surface and gather all the necessary materials for reconstitution and administration (Figure A).
|
- Allow the ADZYNMA and solvent vials to reach room temperature before use.
- Wash and dry your hands thoroughly.
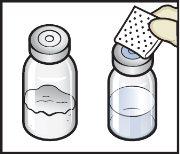
- Remove the plastic caps from the ADZYNMA and solvent vials and place the vials on a flat surface (Figure B).
Figure B
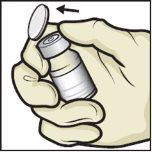
- Clean the rubber stoppers with an alcohol swab and let them dry before use (Figure C).
Figure C
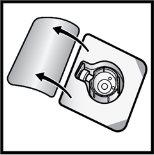
- Open the BAXJECT II Hi-Flow device package by removing the cap, without touching the inside (Figure D).
- Do notremove the BAXJECT II Hi-Flow device from the package.
- Do nottouch the transparent plastic tip.
Figure D
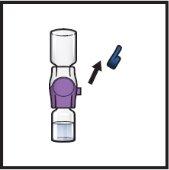
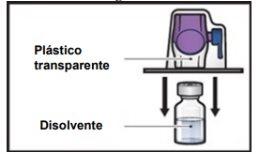
- Turn the package with the BAXJECT II Hi-Flow device upside down and place it over the top of the solvent vial. Press straight down until the transparent plastic tippunctures the solvent vialstopper (Figure E).
Figure E
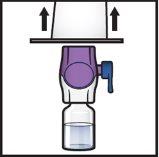
- Hold the BAXJECT II Hi-Flow device package by the edge and remove it from the device (Figure F).
- Do notremove the blue capfrom the BAXJECT II Hi-Flow device.
- Do nottouch the purple plastic tipthat has been exposed.
Figure F
- Turn the system upside downso that the solvent vialis on top. Press the BAXJECT II Hi-Flow device straight down until the purple plastic tippunctures the ADZYNMA powder vialstopper (Figure G). The vacuum will cause the solvent to flow into the ADZYNMA powder vial.
- You may observe bubbles or foam; this is normal and should disappear soon.
Figure G
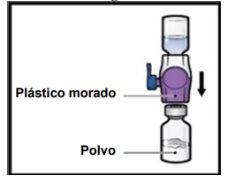
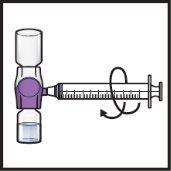
- Gently and continuously turn the connected vials in a circular motion until the powder is completely dissolved (Figure H).
- Do notshake the vial.
Figure H
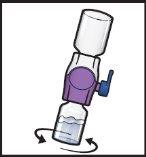
- Before administration, visually inspect the reconstituted solution for particles.
- Do notuse the product if you observe particles or color changes.
- If the dose requires more than one vial of ADZYNMA, reconstitute each vial following the steps above.
- Use a different BAXJECT II Hi-Flow device to reconstitute each ADZYNMA vial.
Administration of ADZYNMA
- Remove the blue capfrom the BAXJECT II Hi-Flow device (Figure I). Connect a Luer-Lock syringe (Figure J).
- Do notinject air into the system.
|
- Turn the system upside down(so that the ADZYNMA vial is on top). Withdraw the reconstituted solutioninto the syringe by slowly pulling the plunger (Figure K).
Figure K
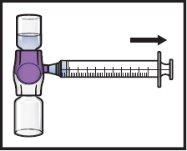
- If a patient is to receive more than one vial of ADZYNMA, the contents of several vials can be withdrawn into the same syringe. Repeat this process with all reconstituted ADZYNMA vials until the total administration volume is reached.
- Disconnect the syringe and connect an appropriate injection needle or infusion set.
- Point the needle upward and eliminate air bubbles by gently tapping the syringe with your finger and slowly and carefully expelling the air from the syringe and needle.
- Apply a tourniquet and clean the chosen injection site with an alcohol swab (Figure L).
Figure L
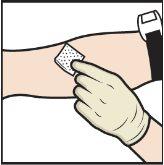
- Insert the needle into the vein and remove the tourniquet.
- Infuse the reconstituted ADZYNMA slowly, at a rate of 2 to 4 ml per minute(Figure M).
- A syringe pump can be used to control the administration rate.
Figure M
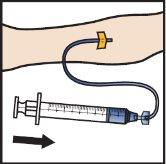
- Remove the needle from the vein and apply pressure to the injection site for several minutes.
- Do notrecap the needle.
Storage of ADZYNMA
- Store ADZYNMA in the refrigerator (between 2°C - 8°C) or at room temperature (up to 30°C) for a period of up to 6 months.
- Do notreturn ADZYNMA to the refrigerator after storage at room temperature.
- Recordthe date ADZYNMA was removed from the refrigerator on the carton.
- Do notfreeze.
- Store in the original packaging to protect from light.
- Do notuse after the expiration date stated on the label and carton after EXP.
- Use ADZYNMA before 3 hourshave passed since reconstitution. Discard the reconstituted medicinal product that has not been used before 3 hours have passed since reconstitution.
Disposal of ADZYNMA
- The vials are for single use.
- Discard the used needle, syringe, and empty vials in a puncture-resistant container for sharp objects.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and any unused medicinal products. This will help protect the environment.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ADZYNMA 1500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTIONDosage form: INJECTABLE, 500 IUActive substance: apadamtase alfa and cinaxadamtase alfaManufacturer: Takeda Manufacturing Austria AgPrescription requiredDosage form: INJECTABLE, 10 mgActive substance: alteplaseManufacturer: Boehringer Ingelheim International GmbhPrescription requiredDosage form: INJECTABLEActive substance: protein CManufacturer: Takeda Manufacturing Austria AgPrescription required
Online doctors for ADZYNMA 1500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Discuss questions about ADZYNMA 1500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions