
ACUOLENS 3 mg/ml + 5.5 mg/ml EYE DROPS IN SINGLE-DOSE CONTAINERS

How to use ACUOLENS 3 mg/ml + 5.5 mg/ml EYE DROPS IN SINGLE-DOSE CONTAINERS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet:information for the user
ACUOLENS 3 mg/ml + 5.5 mg/ml eye drops in single-dose solution
Hypromellose / Sodium chloride
Read the entire leaflet carefully before starting to usethis medication, as it contains important information for you.
Follow the administration instructions for the medication contained in this leaflet or as indicated by your doctor or pharmacist.
- Keep this leaflet, as you may need to read it again.
- If you need advice or more information, consult your pharmacist.
- If you experience side effects, consult your doctor or pharmacist, even if they are side effects not listed in this leaflet. See section 4.
- You should consult a doctor if it worsens or does not improve after 3 days.
Contents of the leaflet
- What is ACUOLENS and what is it used for
- What you need to know before starting to use ACUOLENS
- How to use ACUOLENS
- Possible side effects
- Storage of ACUOLENS
- Contents of the package and additional information
1. What is ACUOLENS and what is it used for
ACUOLENS is an eye drop that, due to its components, hypromellose and sodium chloride, lubricates and protects the cornea, relieving any discomfort (dryness and irritation) caused by decreased tear production.
It is used for the symptomatic relief of irritation and dryness of the eyes.
You should consult a doctor if it worsens or does not improve after 3 days.
2. What you need to know before starting to use ACUOLENS
Do not use ACUOLENS
- if you are allergic to hypromellose, sodium chloride, or any of the other components of this medication (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist before starting to use ACUOLENS.
Use this medication only in your eye(s).
If you experience headache, eye pain, changes in vision, eye irritation, continued redness, or if symptoms worsen or persist for more than 3 days, discontinue treatment and consult your doctor.
Children
No specific studies have been conducted in children. Consult your doctor before using this medication in children.
Other medications and ACUOLENS
Inform your doctor or pharmacist if you are using, have recently used, or may need to use any other medication.
Pregnancy, breastfeeding, and fertility
Consult your doctor before using any medication.
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
This medication can be used during pregnancy and breastfeeding.
Driving and using machines
Like other eye drops, immediately after applying this medication, you may notice that your vision becomes blurry. Do not drive or use machines until your vision is clear.
ACUOLENS contains phosphate buffer
This medication contains 2 mg of phosphates in each 0.5 ml.
If you have severe corneal damage (the transparent layer on the front of the eye), treatment with phosphates, in very rare cases, can cause blurry vision due to calcium accumulation.
3. How to use ACUOLENS
Follow the administration instructions for the medication contained in this leaflet or as indicated by your doctor or pharmacist. In case of doubt, ask your doctor or pharmacist.
The recommended dose is:
Adults (including elderly patients)
Instill 1 or 2 drops into each affected eye, as needed.
The number of daily applications and the duration of treatment may be modified according to medical criteria.
Recommendations for use:

1 2 3
- Open the aluminum pouch and take the single-dose strip.
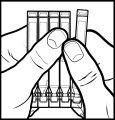
- Hold the strip with the smooth long end facing up and separate one of the single doses by pulling it towards you while keeping the others firmly held. You will need to detach the points where it is attached to the other single doses (figure 1).
- Keep the separated single dose. Put the rest of the single doses back in the aluminum pouch.
- Make sure you have a mirror and wash your hands.
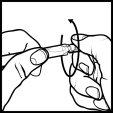
- Hold the smooth long end of the single dose between your fingers and open it by twisting the other end (figure 2).
- Tilt your head back. Gently separate the lower eyelid from the eye with a finger, until a pocket forms, where the drop should fall (figure 3).
- Hold the single dose between your fingers with the open tip facing down.
- Bring the tip of the container close to the eye. A mirror may be helpful.
- Do not touch the eye, eyelid, or surrounding areas with the tip of the container. The drops may become contaminated.
- Gently squeeze the container to release one drop into the pocket formed between the eyelid and the eye.
- If you are applying drops to both eyes, repeat the previous steps for the other eye, using the same container.
- Discard the container and any remaining solution immediately.
- Discard any unused container one week after opening the aluminum pouch, even if the containers are still unopened.
If a drop falls outside the eye, try again.
If you are using other eye medications, wait at least 5 minutes between the administration of this eye drop and other eye medications. Eye ointments should be administered last.
If you use more ACUOLENS than you should, you can eliminate it by rinsing your eyes with warm water.
Due to the characteristics of this preparation, intoxication phenomena are not expected with the ocular use of the product.
In case of overdose or accidental ingestion, consult your doctor or call the Toxicology Information Service immediately, phone 91 562 04 20, indicating the medication and the amount used.
If you forget to use ACUOLENS, do not worry, apply the drops as soon as possible. Do not apply a double dose to make up for forgotten doses.
If you have any other questions about using this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone experiences them.
Observed side effects.
Frequency not known(frequency cannot be estimated from available data):
- Eye effects: blurred vision, eye pain, abnormal sensation in the eye, eye irritation, eye redness, eye allergy, and eye itching
- Body effects: allergic reaction (hypersensitivity)
Reporting side effects
If you experience any type of side effect, consult your doctor or pharmacist, even if it is a possible side effect not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medications: https://www.notificaRAM.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of ACUOLENS
Keep this medication out of sight and reach of children.
No special storage conditions are required.
Do not use this medication after the expiration date shown on the single dose and on the box after "EXP". The expiration date is the last day of the month indicated.
You must discard the single dose as soon as you have used it.Once the aluminum pouch is opened, discard any unused single doses one week after opening.
Medications should not be thrown down the drain or into the trash. Deposit the containers and medications you no longer need at the SIGRE collection point in the pharmacy. If in doubt, ask your pharmacist how to dispose of the containers and medications you no longer need. This will help protect the environment.
6. Contents of the package and additional information
Composition of ACUOLENS
- The active ingredients are hypromellose and sodium chloride. One ml of solution contains 3 mg of hypromellose and 5.5 mg of sodium chloride.
- The other components are potassium chloride, magnesium chloride hexahydrate, calcium chloride dihydrate, zinc chloride, disodium hydrogen phosphate dodecahydrate, sodium phosphate monobasic monohydrate, sodium hydrogen carbonate, and purified water.
Appearance of the product and contents of the package
This medication is a liquid (clear and colorless) that comes in a box of 30 single-dose plastic containers of 0.5 ml.
Marketing authorization holder and manufacturer
Marketing authorization holder
Alcon Healthcare S.A.
World Trade Center Almeda Park
Plaça de la Pau s/n, Edificio 6, planta 3
08940 - Cornellà de Llobregat (Barcelona)
Spain
Manufacturer
Alcon-Couvreur N.V.
Rijksweg 14
B-2870 Puurs
Belgium
or
Alcon Laboratories Belgium
Lichterveld 3
2870 Puurs-Sint-Amands
Belgium
Date of the last revision of this leaflet: August 2022
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price8.6 EUR
- Active substance
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ACUOLENS 3 mg/ml + 5.5 mg/ml EYE DROPS IN SINGLE-DOSE CONTAINERSDosage form: EYEDROP, 3.2 mg/mlActive substance: artificial tears and other indifferent preparationsManufacturer: Bausch & Lomb S.A.Prescription not requiredDosage form: EYE DROP, 3.2 mg/mlActive substance: artificial tears and other indifferent preparationsManufacturer: Bausch & Lomb S.A.Prescription not requiredDosage form: EYEDROP, 5 mg/mlActive substance: artificial tears and other indifferent preparationsManufacturer: Neuraxpharm Spain S.L.Prescription not required
Online doctors for ACUOLENS 3 mg/ml + 5.5 mg/ml EYE DROPS IN SINGLE-DOSE CONTAINERS
Discuss questions about ACUOLENS 3 mg/ml + 5.5 mg/ml EYE DROPS IN SINGLE-DOSE CONTAINERS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















