
THEO-DUR 200 mg PROLONGED-RELEASE TABLETS

How to use THEO-DUR 200 mg PROLONGED-RELEASE TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
THEO-DUR 200mg prolonged-release tablets
Theophylline
Read this package leaflet carefully before you start taking this medicine, because it contains important information for you.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. See section 4.
Contents of the package leaflet
- What Theo-Dur is and what it is used for
- What you need to know before you take Theo-Dur
- How to take Theo-Dur
- Possible side effects
- Storage of Theo-Dur
- Contents of the pack and other information
1. What Theo-Dur is and what it is used for
This medicine belongs to a group of medicines called bronchodilators, which means it dilates the bronchi and facilitates breathing.
It is indicated for the prevention and treatment of asthma or bronchial spasm associated with lung diseases such as chronic bronchitis or emphysema.
2. What you need to know before you take Theo-Dur
Do not take Theo-Dur
- If you are allergic to the active substance or to any other medicine of the same group or to any of the other components of this medicine (listed in section 6).
- If you have acute heart rhythm disorders (tachyarrhythmia).
- If you have recently had a heart attack.
Warnings and precautions
Talk to your doctor or pharmacist before taking Theo-Dur if:
- You have any heart disease,
- You have high blood pressure (hypertension),
- You have an overactive thyroid gland (hyperthyroidism),
- You have seizure disorders (epilepsy),
- You have stomach ulcers (gastric and/or duodenal),
- You have hemoglobin production deficiencies (porphyria),
- You have liver and/or kidney problems (hepatic and/or renal insufficiency),
- You know you do not eliminate theophylline correctly.
Children
The use of theophylline is not recommended in children under one year of age.
Taking Theo-Dur with other medicines
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Theophylline may decrease or increase the effect of other medicines, and in turn, the effect of theophylline may be decreased or increased by other medicines if taken together. For this reason, it is necessary that you inform your doctor if you are taking:
- Other preparations containing theophylline or other xanthines (such as caffeine and similar substances),
- Beta2 adrenergic receptor inducers (beta2-agonists, generally used to treat asthma),
- Barbiturates, especially phenobarbital, pentobarbital, and primidone (used for sedation, relaxation),
- Carbamazepine (anticonvulsant used for epilepsy),
- Phenytoin and fosphenytoin (used for epilepsy),
- Rifampicin and rifapentine (antibiotics),
- Sulfinpyrazone (used to treat gout),
- Products containing hypericum (St. John's Wort),
- Oral contraceptives,
- Macrolide antibiotics (especially erythromycin, troleandomycin, clarithromycin, josamycin, and spiramycin),
- Quinolone antibiotics (gyrase inhibitors, especially ciprofloxacin, enoxacin, and pefloxacin; see below),
- The antibiotic imipenem (especially central nervous system side effects such as seizures),
- Isoniazid (treatment of tuberculosis),
- Thiabendazole (treatment of fungi),
- Calcium channel blockers (e.g., verapamil and diltiazem) used for heart disease,
- Propranolol (treatment of high blood pressure),
- Mexiletine (treatment of heart problems),
- Propafenone (treatment of heart problems),
- Ticlopidine (prevention of blood coagulation),
- Cimetidine, ranitidine, etintidine (preventing acid production in the stomach),
- Allopurinol, febuxostat (treatment of gout),
- Fluvoxamine (treatment of mental illnesses),
- Interferon alfa and peginterferon alfa-2 (treatment of immunological disorders),
- Zafirlukast (treatment of asthma),
- Influenza vaccines,
- Hydrocortisone (muscle treatments),
- Zileuton (treatment of asthma),
- Diuretics (furosemide),
- Halothane (general anesthetic),
- Digitalis (treatment of heart problems),
- Benzodiazepines (used for sedation, relaxation).
If you are a smoker, tell your doctor, as it may be necessary to change your dose.
Interaction with diagnostic tests
If you are going to undergo any diagnostic tests (including blood tests, urine tests, skin tests using allergens), inform your doctor that you are taking this medicine, as it may alter the results.
Taking Theo-Dur with food and drinks
High consumption of caffeine-containing beverages such as tea, coffee, cocoa, cola, and large amounts of chocolate should be avoided. These products can increase the side effects of this medicine.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to become pregnant, consult your doctor before using this medicine.
Pregnancy
The use of theophylline is not recommended during pregnancy.
Breastfeeding
The use of theophylline is not recommended during breastfeeding.
Elderly people
Do not use in people over 65 years old without consulting a doctor.
Driving and using machines
This type of medicine can alter reaction speed, so you should be careful when driving and/or using machines, especially at the start of treatment, when changing the dose, or when taking it with other medicines.
Theo-Dur contains lactose
If your doctor has told you that you have an intolerance to some sugars, consult with them before taking this medicine.
Theo-Dur contains sucrose
If your doctor has told you that you have an intolerance to some sugars, consult with them before taking this medicine.
3. How to take Theo-Dur
Follow exactly the administration instructions of this medicine indicated by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again.
Your doctor will indicate the exact dose you should take. The doses will be taken at regular 12-hour intervals. It is important to strictly follow the dosage regimen, especially regarding the spacing of doses.
As a guideline, the following dose can be followed, but remember that the dose should be as recommended by your doctor.
Adults and adolescents over 16 years
The recommended dose for adults is 200 mg (1 tablet) every 12 hours. Your doctor may increase the dose depending on how you respond to treatment.
Pediatric population (between 1 and 16 years)
The recommended dose is 4 mg per kilogram of body weight per day. This amount will be divided into two doses every 12 hours.
Elderly patients
In the case of elderly patients, your doctor will assess the dose to be administered.
Method of administration
The tablets should be taken without dissolving, without chewing or biting, swallowing them with sufficient liquid.
It is recommended to take it at night, before going to bed.
If you take more Theo-Dur than you should
Consult your doctor or the nearest emergency service or call the Toxicology Information Service, Phone 915 620 420, indicating the medicine and the amount taken. Bring the packaging and any remaining tablets with you.
If you forget to take Theo-Dur
Do not take a double dose to make up for forgotten doses. Take the next dose as your doctor has indicated, at the usual time.
If you stop taking Theo-Dur
If you stop your treatment with Theo-Dur, your disease may worsen. Do not stop taking the medicine unless your doctor tells you to.
If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The possible side effects are listed below:
- Tachycardia and arrhythmia (changes in heartbeats), palpitations, drop in blood pressure.
- Gastrointestinal problems, nausea, vomiting, diarrhea, stomach problems (weakness of the muscular tone in the lower esophageal sphincter) that can increase nocturnal esophageal reflux if you already have it.
- Allergic reactions (hypersensitivity).
- Blood disorders such as: decreased potassium (hypokalemia), increased calcium (hypercalcemia), increased sugar (hyperglycemia), increased creatinine, increased uric acid (hyperuricemia), and changes in electrolytes.
- Headache, excitement, tremors, nervousness, insomnia, seizures.
- Increased urine production (diuresis).
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if it is possible side effects not listed in this package leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines (website: www.notificaRAM.es).
By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Theo-Dur
Keep this medicine out of the sight and reach of children.
No special storage conditions are required.
Do not use this medicine after the expiry date stated on the packaging after EXP. The expiry date is the last day of the month indicated.
Medicines should not be disposed of via wastewater or household waste. Return any unused medicine to a pharmacy for disposal. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
Composition of Theo-Dur
- The active substance is theophylline. Each tablet contains 200 mg of anhydrous theophylline.
- The other components are: acetylcellulose, cetyl alcohol, myristyl alcohol, white wax, diethyl phthalate, magnesium stearate, hydroxypropyl methylcellulose, anhydrous lactose, glycerol monostearate, neutral pellets (cornstarch and sugar spheres [sucrose and cornstarch]).
Appearance of the product and contents of the pack
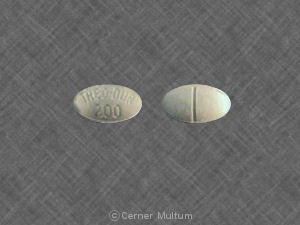
Theo-Dur 200 mg prolonged-release tablets are presented in the form of white, cylindrical, scored tablets. The packs contain 40 tablets.
Marketing authorization holder and manufacturer
Marketing authorization holder
VEGAL FARMACEUTICA, S.L.
Vía de las Dos Castillas 9C, portal 2, 2º C
28224 Pozuelo de Alarcón – Madrid. Spain.
Manufacturer
GENERIS FARMACÊUTICA, S.A.
Rua Joäo de Deus, 19, Venda Nova, 2700-487 Amadora – Portugal
or
COVEX, S.A.
Calle Acero 25, Polígono Industrial Sur
28770 Colmenar Viejo - Madrid. Spain
Other presentations
Theo-Dur 100 mg prolonged-release tablets: Pack of 40 tablets.
Theo-Dur 300 mg prolonged-release tablets: Pack of 40 tablets.
Date of last revision of this package leafletSeptember 2013
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price4.54 EUR
- Availability in pharmacies
Supply issue reported
Data from the Spanish Agency of Medicines (AEMPS) indicates a supply issue affecting this medicine.<br><br>Availability may be limited in some pharmacies.<br><br>For updates or alternatives, consult your pharmacist. - Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to THEO-DUR 200 mg PROLONGED-RELEASE TABLETSDosage form: ORAL SOLUTION/SUSPENSION, 5.33 mg anhydrous theophyllineActive substance: theophyllineManufacturer: Tora Laboratories S.L.U.Prescription requiredDosage form: MODIFIED-RELEASE TABLET, 300 mgActive substance: theophyllineManufacturer: Laboratorio Aldo Union S.L.Prescription requiredDosage form: MODIFIED-RELEASE TABLET, 100 mgActive substance: theophyllineManufacturer: Vegal Farmaceutica S.L.Prescription required
Online doctors for THEO-DUR 200 mg PROLONGED-RELEASE TABLETS
Discuss questions about THEO-DUR 200 mg PROLONGED-RELEASE TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















