STALEVO 200 mg/50 mg/200 mg FILM-COATED TABLETS

How to use STALEVO 200 mg/50 mg/200 mg FILM-COATED TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Stalevo 200mg/50mg/200mg film-coated tablets
Levodopa/carbidopa/entacapone
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Stalevo and what is it used for
- What you need to know before you take Stalevo
- How to take Stalevo
- Possible side effects
- Storing Stalevo
- Contents of the pack and other information
1. What is Stalevo and what is it used for
Stalevo contains three active substances (levodopa, carbidopa and entacapone) in one film-coated tablet. Stalevo is used to treat Parkinson's disease.
Parkinson's disease is due to a lack of dopamine in the brain. Levodopa increases the amount of dopamine and thereby reduces the symptoms of Parkinson's disease. Carbidopa and entacapone improve the antiparkinsonian effects of levodopa.
2. What you need to know before you take Stalevo
Do not take Stalevo if:
- you are allergic to levodopa, carbidopa or entacapone, or any of the other ingredients of this medicine (listed in section 6)
- you have narrow-angle glaucoma (a condition where the pressure in the eye is increased)
- you have a tumor of the adrenal gland
- you are taking certain medicines for depression (combinations of selective MAO-A and MAO-B inhibitors, or non-selective MAO inhibitors)
- you have ever had neuroleptic malignant syndrome (NMS - a rare reaction to medicines used to treat severe mental disorders)
- you have ever had non-traumatic rhabdomyolysis (a rare muscle disorder)
- you have severe liver disease.
Warnings and precautions
Tell your doctor or pharmacist before you start taking Stalevo if you have or have had:
- a heart attack or any other heart disease, including arrhythmias, or blood vessel disease
- asthma or any other lung disease
- any liver problem, as you may need to have your dose adjusted
- any kidney disease or hormonal disorder
- stomach ulcers or convulsions
- prolonged diarrhea. Check with your doctor as it may be a sign of colon inflammation
- any severe mental disorder, such as psychosis
- open-angle glaucoma, as your dose may need to be adjusted and your eye pressure monitored.
Tell your doctor if you are taking:
- antipsychotics (medicines for treating psychosis)
- any medicine that may cause a drop in blood pressure when standing up from a chair or bed. You should be aware that Stalevo may worsen these reactions.
Tell your doctor if, while taking Stalevo, you:
- notice great stiffness in your muscles or that they contract violently, or if you suffer from tremors, agitation, confusion, fever, rapid heartbeat or large fluctuations in blood pressure. If any of these occur, contact your doctor immediately
- feel depressed, have suicidal thoughts or notice unusual changes in your behavior
- find that you suddenly fall asleep, or feel very drowsy. If this happens, do not drive or use tools or machines (see also section “Driving and using machines”)
- start to notice uncontrolled movements or if they get worse. If this happens, your doctor may need to adjust your dose of medication
- suffer from diarrhea: it is recommended to monitor your weight to avoid excessive weight loss
- suffer from progressive loss of appetite, asthenia (weakness, exhaustion) and weight loss over a relatively short period. In these cases, a complete medical evaluation, including a liver function test, should be considered
- consider that you need to stop taking Stalevo; consult the section "If you stop taking Stalevo".
Tell your doctor if you, or your family member/caregiver, notice that you are developing symptoms similar to addiction, leading to a desire for high doses of Stalevo and other medicines used to treat Parkinson's disease.
Tell your doctor if you, or your family member/caregiver, notice that you are developing desires or tendencies to behave in an unusual way, or that you cannot resist the impulse, determination or temptation to perform certain activities that may harm you or others. These behaviors are called impulse control disorders and may include addiction to gambling, eating or spending, and an abnormally high sexual drive or preoccupation with increased thoughts or feelings of sex. Your doctor may need to review your treatment.
During prolonged treatment with Stalevo, your doctor may perform regular blood tests.
If you need to undergo surgery, tell your doctor that you are taking Stalevo.
Stalevo is not recommended for the treatment of extrapyramidal symptoms (e.g. involuntary movements, agitation, muscle stiffness and contractions) caused by other medicines.
Children and adolescents
Experience with the use of Stalevo in patients under 18 years is limited. Therefore, it is not recommended for use in children or adolescents.
Taking Stalevo with other medicines
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Do not take Stalevo if you are taking certain medicines for depression (combinations of selective MAO-A and MAO-B inhibitors, or non-selective MAO inhibitors).
Stalevo may increase the effect, and also the side effects, of certain medicines. These include:
- medicines used to treat depression, such as moclobemide, amitriptyline, desipramine, maprotiline, venlafaxine and paroxetine
- rimiterol and isoprenaline, used to treat respiratory diseases
- adrenaline, used for severe allergic reactions
- noradrenaline, dopamine and dobutamine, used to treat heart diseases and low blood pressure
- alpha-methyldopa, used to treat high blood pressure
- apomorphine, used to treat Parkinson's disease.
Certain medicines may reduce the effects of Stalevo. These medicines are:
- dopamine antagonists used to treat mental disorders, nausea and vomiting
- phenytoin, used to prevent convulsions
- papaverine, used to relax muscles.
Stalevo may interfere with the absorption of iron from the gut. Therefore, do not take Stalevo at the same time as iron supplements. If you take any, wait at least 2 or 3 hours before taking the other.
Taking Stalevo with food and drinks
- Stalevo can be taken with or without food.
- It is possible that in some patients Stalevo may not be absorbed well if taken with protein-rich foods (meat, fish, dairy products, cereals and nuts) or shortly after eating them. Consult your doctor if you think this is happening to you.
Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
You should not breast-feed while taking Stalevo.
Driving and using machines
Stalevo may lower your blood pressure, which may cause dizziness or fainting. Therefore, be careful when using tools or machines.
If you feel very tired or notice that you suddenly fall asleep, wait until you feel fully awake before driving or doing anything that requires you to be alert. Otherwise, you may put yourself and others at risk of a serious or even fatal accident.
Stalevo contains sucrose
Stalevo contains sucrose (2.3 mg per tablet). If your doctor has told you that you have an intolerance to some sugars, consult them before taking this medicine.
3. How to take Stalevo
Always take this medicine exactly as your doctor or pharmacist has told you. If you are not sure, check with your doctor or pharmacist.
Adults and the elderly:
- Your doctor will tell you exactly how many Stalevo tablets to take each day.
- The tablets must not be broken or divided into smaller pieces.
- Take only one tablet at a time.
- Depending on how you respond to treatment, your doctor may ask you to increase or decrease the dose.
- If you are taking Stalevo 200 mg/50 mg/200 mg tablets, do not take more than 7 tablets per day.
Talk to your doctor or pharmacist if you think the effect of Stalevo is too strong or too weak, or if you experience possible side effects.
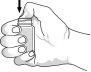
To open the bottle for the first time: open the cap by pressing with your thumb on the seal until it breaks. See Figure 1. | Figure 1
|
If you take more Stalevo than you should
If you accidentally take more Stalevo tablets than you should, talk to your doctor or pharmacist immediately. In case of overdose, you may feel dizzy or agitated, your heart rate may be slower or faster than normal, or your skin, tongue, eyes or urine may change color.
If you forget to take Stalevo
Do not take a double dose to make up for forgotten doses.
If it is less than 1 hour until your next dose:
Take a tablet as soon as you remember, and the next one at the usual time.
If it is more than 1 hour until your next dose:
Take a tablet as soon as you remember, and the next one 1 hour later. Then continue as usual.
There should always be 1 hour between Stalevo tablets to avoid possible side effects.
If you stop taking Stalevo
Do not stop taking Stalevo unless your doctor tells you to. If your doctor tells you to stop taking Stalevo, they may need to adjust the dose of your other Parkinson's medicines, especially levodopa, to control your symptoms. If you stop taking Stalevo and other Parkinson's medicines suddenly, you may experience unwanted side effects.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. Many can be relieved by adjusting the dose.
If you experience any of these symptoms while taking Stalevo, contact your doctor immediately:
- Great stiffness or violent contractions of the muscles, tremors, agitation, confusion, fever, rapid heartbeat or large fluctuations in blood pressure. These may be symptoms of neuroleptic malignant syndrome (NMS - a rare and serious reaction to medicines used to treat disorders of the central nervous system) or rhabdomyolysis (a rare and serious muscle disorder).
- An allergic reaction characterized by signs such as hives, itching, rash and swelling of the face, lips, tongue or throat. This reaction may cause difficulty breathing or swallowing.
Very common (may affect more than 1 in 10 people):
- uncontrolled movements (dyskinesias)
- feeling sick (nausea)
- brownish discoloration of urine (harmless)
- muscle pain
- diarrhea
Common (may affect up to 1 in 10 people):
- fainting or fainting due to low blood pressure, high blood pressure
- worsening of parkinsonian symptoms, dizziness, drowsiness
- vomiting; abdominal pain and discomfort; heartburn; dry mouth; constipation
- sleeplessness; hallucinations; confusion; abnormal dreams (with nightmares); fatigue
- changes in mental state, including memory problems, anxiety and depression (possibly with suicidal thoughts)
- heart or blood vessel disorders (e.g. chest pain), irregular heartbeats
- more frequent falls
- breathlessness
- increased sweating, rash
- muscle cramps, swelling of the legs
- blurred vision
- anemia
- decreased appetite, weight loss
- headache, joint pain
- urinary tract infections
Uncommon (may affect up to 1 in 100 people):
- heart attack
- intestinal bleeding
- changes in blood cell counts that may cause bleeding; abnormalities in liver function tests
- seizures
- feeling agitated
- psychotic symptoms
- colitis (inflammation of the colon)
- color changes other than those of urine (e.g. skin, nails, hair, sweat)
- difficulty swallowing
- inability to urinate
Frequency not known (cannot be estimated from the available data):
Desire for high doses of Stalevo, higher than those required to control motor symptoms, known as dopamine dysregulation syndrome. Some patients experience severe abnormal involuntary movements (dyskinesias), mood changes or other side effects after taking high doses of Stalevo.
The following side effects have also been reported:
- hepatitis (inflammation of the liver)
- itching
You may experience the following side effects:
- Inability to resist the impulse to perform an action that could be harmful, which may include:
- a strong impulse to gamble excessively despite severe personal or family consequences
- an increased or altered interest in sex and a disturbing behavior for you or others, such as an increased sexual drive
- excessive or uncontrolled shopping or spending
- eating excessively (eating large amounts of food in short periods) or compulsively (eating more food than usual and more than needed to satisfy hunger).
Tell your doctor if you experience any of these behaviors: they will discuss how to manage or reduce these symptoms.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Annex V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Stalevo
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton after EXP. The expiry date refers to the last day of the month stated.
No special storage conditions are required.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Packaging Contents and Additional Information
Composition of Stalevo
- The active ingredients of Stalevo are levodopa, carbidopa, and entacapone.
- Each Stalevo 200 mg/50 mg/200 mg tablet contains 200 mg of levodopa, 50 mg of carbidopa, and 200 mg of entacapone.
- The other components of the tablet core are sodium croscarmellose, magnesium stearate, corn starch, mannitol (E 421), and povidone (E 1201).
- The components of the film coating are glycerol 85 percent (E 422), hypromellose, magnesium stearate, polysorbate 80, red iron oxide (E 172), sucrose, and titanium dioxide (E 171).
Appearance of the Product and Packaging Contents
Stalevo 200 mg/50 mg/200 mg: oval, uncoated, film-coated tablets, dark reddish-brown in color with “LCE 200” engraved on one side.
There are five different packaging sizes for Stalevo 200 mg/50 mg/200 mg (10, 30, 100, 130, or 175 tablets). Not all packaging sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Orion Corporation
Orionintie 1
FI-02200 Espoo
Finland
You can request more information about this medication from the local representative of the marketing authorization holder.
Belgium/België/Belgien Orion Pharma BVBA/SPRL Tel: +32 (0)15 64 10 20 | Lithuania UAB Orion Pharma Tel: +370 5 276 9499 |
Greece Orion Corporation Tel: +358 10 4261 | Luxembourg/Luxemburg Orion Pharma BVBA/SPRL Tel: +32 (0)15 64 10 20 |
Czech Republic Orion Pharma s.r.o. Tel: +420 234 703 305 | Hungary Orion Pharma Kft. Tel: +36 1 239 9095 |
Denmark Orion Pharma A/S Tel: +45 8614 0000 | Malta Orion Corporation Tel: +358 10 4261 |
Germany Orion Pharma GmbH Tel: +49 40 899 6890 | Netherlands Orion Pharma BVBA/SPRL Tel: +32 (0)15 64 10 20 |
Estonia Orion Pharma Eesti OÜ Tel: +372 66 44 550 | Norway Orion Pharma AS Tel: +47 40 00 42 10 |
Greece Orion Pharma Hellas M.E.Π.E. Tel: +30 210 980 3355 | Austria Orion Pharma GmbH Tel: +49 40 899 6890 |
Spain Orion Pharma S.L. Tel: +34 91 599 86 01 | Poland Orion Pharma Poland Sp. z o.o. Tel: +48 22 8333177 |
France Centre Spécialités Pharmaceutiques Tel: +33 (0)1 47 04 80 46 | Portugal Orionfin Unipessoal Lda Tel: +351 21 154 68 20 |
Croatia Orion Pharma d.o.o. Tel: +386 (0)1 600 8015 | Romania Orion Corporation Tel: +358 10 4261 |
Ireland Orion Pharma (Ireland) Ltd. c/o Allphar Services Ltd. Tel: +353 1 428 7777 | Slovenia Orion Pharma d.o.o. Tel: +386 (0)1 600 8015 |
Iceland Vistor hf. Tel: +354 535 7000 | Slovakia Orion Pharma s.r.o. Tel: +420 234 703 305 |
Italy Orion Pharma S.r.l. Tel: +39 02 67876111 | Finland Orion Corporation Tel: +358 10 4261 |
Cyprus Lifepharma (ZAM) Ltd Tel: +357 22056300 | Sweden Orion Pharma AB Tel: +46 8 623 6440 |
Latvia Orion Corporation Orion Pharma parstavnieciba Tel: +371 20028332 | United Kingdom Orion Pharma (UK) Ltd. Tel: +44 1635 520 300 |
Date of the Last Revision of this Leaflet:
Other Sources of Information
Detailed information about this medication is available on the European Medicines Agency website http://www.ema.europa.eu
- Country of registration
- Average pharmacy price80.72 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to STALEVO 200 mg/50 mg/200 mg FILM-COATED TABLETSDosage form: INTESTINAL GEL, 20 mg/ml + 5 mg/ml + 20 mg/mlActive substance: levodopa, decarboxylase inhibitor and COMT inhibitorManufacturer: Lobsor Pharmaceuticals AbPrescription requiredDosage form: TABLET, 100 mg/25 mg/200 mgActive substance: levodopa, decarboxylase inhibitor and COMT inhibitorManufacturer: Abdi Farma GmbhPrescription requiredDosage form: TABLET, 125mg/31.25mg/200 mgActive substance: levodopa, decarboxylase inhibitor and COMT inhibitorManufacturer: Abdi Farma GmbhPrescription required
Online doctors for STALEVO 200 mg/50 mg/200 mg FILM-COATED TABLETS
Discuss questions about STALEVO 200 mg/50 mg/200 mg FILM-COATED TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions