PENTASA 4 g RECTAL SUSPENSION


How to use PENTASA 4 g RECTAL SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Pentasa 4 g Rectal Suspension
Mesalazine
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information
- What Pentasa rectal suspension is and what it is used for
- What you need to know before you use Pentasa rectal suspension
- How to use Pentasa rectal suspension
- Possible side effects
- Storage of Pentasa rectal suspension
- Contents of the pack and further information
1. What Pentasa rectal suspension is and what it is used for
Pentasa rectal suspension is indicated for the treatment of ulcerative colitis localized in the rectum and sigmoid colon.
Ulcerative colitis is an inflammatory bowel disease in which the lining of the intestine is inflamed and develops many small breaks in its surface (ulcers) that can bleed.
Pentasa contains the active substance mesalazine, which belongs to a group of medicines called intestinal anti-inflammatory agents that help reduce inflammation and painful symptoms.
2. What you need to know before you use Pentasa rectal suspension
Do not use PENTASA rectal suspension:
- if you are allergic to the active substance or to any of the other ingredients of this medicine (listed in section 6)
- if you are allergic to salicylates, e.g. aspirin
- if you have severe kidney and/or liver problems
Warnings and precautions
Consult your doctor or pharmacist before starting to use Pentasa rectal suspension:
- if you are allergic to sulfasalazine (risk of allergy to salicylates)
- if you currently have or have had liver or kidney function impairment
- if you have a disease that may make you prone to bleeding
- if you are undergoing treatment that may affect kidney function, e.g. non-steroidal anti-inflammatory drugs (NSAIDs), such as aspirin
- if you have respiratory problems, in particular asthma
- treatment should be discontinued immediately in case of acute symptoms of intolerance, e.g. cramps, abdominal pain, fever, severe headache, and rash.
- Kidney stones may occur with the use of mesalazine. Symptoms include pain in the sides of the abdomen and blood in the urine. Make sure to drink a sufficient amount of liquid during treatment with mesalazine.
- If you have ever suffered from a severe skin rash or skin peeling, blisters, or sores in the mouth after using mesalazine
Mesalazine may cause a discoloration of the urine to a reddish-brown color after contact with sodium hypochlorite bleach in the toilet water. This is a chemical reaction between mesalazine and bleach and is harmless.
Be careful with mesalazine:
Severe skin reactions, such as drug reaction with eosinophilia and systemic symptoms (DRESS), Stevens-Johnson syndrome (SSJ), and toxic epidermal necrolysis (NET), have been observed with mesalazine treatment. Stop taking mesalazine and seek medical attention immediately if you notice any of the symptoms related to these severe skin reactions described in section 4.
If you experience severe or recurrent headache, vision changes, or ringing or buzzing in the ears, contact your doctor immediately.
While you are being treated with this medicine, your doctor will perform blood and urine tests to monitor your kidney function, especially at the start of treatment.
Use in children:
There is limited documentation on the effect in children (6-18 years).
Use in elderly:
It should be used with caution in the elderly and only in patients with normal kidney function.
Use of Pentasa with other medicines:
Tell your doctor or pharmacist if you are using or have recently used or might use any other medicines.
This is especially important if you are taking any of the following medicines:
- azathioprine (used after transplants or to treat autoimmune diseases)
- 6-mercaptopurine or thioguanine (chemotherapy, used to treat leukemia)
- certain drugs that inhibit blood coagulation (medicines for thrombosis or to thin your blood)
Pentasa 4 grams rectal suspension with food and drinks
Not applicable.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, or think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
There is limited experience with the use of mesalazine during pregnancy and breastfeeding.
Blood disorders have been observed in newborns of mothers treated with this medicine.
Newborns may develop allergic reactions after breastfeeding, e.g. diarrhea. If the newborn has diarrhea, breastfeeding should be discontinued.
Fertility:
Data on mesalazine in animals show that it has no effect on male or female fertility.
Driving and using machines:
Treatment with Pentasa rectal suspension does not appear to affect the ability to drive or use machines.
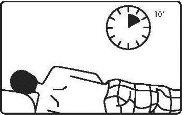
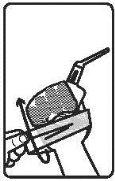
3. How to use Pentasa 4 grams rectal suspension
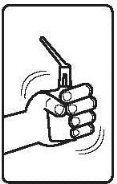
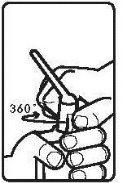
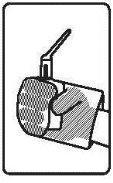
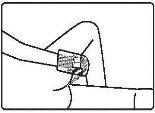
Follow the instructions for administration of this medicine exactly as indicated by your doctor. If you are unsure, consult your doctor or pharmacist again. Remember to use your medicine.
The rectal suspension sachets are protected by an aluminum bag and should be used immediately after opening.
It is recommended to evacuate before administration of the rectal suspension.
Instructions for use
|
|
|
|
|
|
|
|
Note: It is recommended to protect bedding from possible spills, as Pentasa rectal suspension may discolor fabrics. If Pentasa rectal suspension accidentally spills on fabric, soak it immediately.
The recommended dose is:
Adults: 1 sachet of rectal suspension of 100 milliliters (4 grams) at bedtime for 2-3 weeks. The dose may be reduced depending on the patient's body weight.
Children (6-18 years): There is limited experience and documentation on the effect in children.
Dosage should be adjusted according to the patient's response.
If you use more Pentasa rectal suspension than you should
No cases of overdose have been reported in humans, but if an overdose is suspected, consult a doctor.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount ingested.
If you stop treatment with Pentasa rectal suspension
Your doctor will tell you how long to use Pentasa rectal suspension. Do not stop treatment before, even if you feel better, as symptoms may return if treatment is stopped too soon. Follow your doctor's instructions strictly for the duration of treatment.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Pentasa rectal suspension can cause side effects, although not everybody gets them.
Severe side effects:
Very few cases of severe allergic reaction (including severe skin reactions that can affect the skin as a protective barrier of the body) have been reported. The allergic reaction could lead to swelling of the face and neck and/or difficulty breathing or swallowing (angioedema). If this happens, contact your doctor or emergency services immediately.
Tell your doctor immediately if you experience severe or recurrent headache, vision changes, or ringing or buzzing in the ears. These could be symptoms of increased pressure inside your skull (idiopathic intracranial hypertension).
Stop taking mesalazine and seek medical attention immediately if you experience any of the following symptoms:
- red patches, or circular or coin-shaped patches on the chest, often with central blisters, skin peeling, ulcers in the mouth, throat, nose, genitals, and eyes, generalized rash, fever, and swollen lymph nodes. These severe skin reactions are often preceded by fever or flu-like symptoms.
The following frequent side effectsaffect between 1 and 10 in every 100 patients treated:
- headache
- diarrhea
- abdominal pain
- nausea
- vomiting
- skin rash
- flatulence (gas)
- local reactions such as itching, rectal discomfort, and urge to defecate
- Anal discomfort and irritation at the site of administration, itching, and feeling of needing to defecate.
The following rare side effectsaffect between 1 and 10 in every 10,000 patients treated:
- inflammation of certain areas of the heart (myocarditis and pericarditis) that can cause difficulty breathing and chest pain or palpitations (rapid or irregular heartbeat)
- inflammation of the pancreas (including symptoms of back and/or stomach pain) and increased amylase
- dizziness
- increased sensitivity of the skin to sunlight and ultraviolet light (photosensitivity)
The following very rare side effectsaffect less than 1 in 10,000 patients treated:
- eosinophilia (as part of an allergic reaction) and blood disorders such as reduction of red blood cells (anemia), white blood cells (leukopenia), or platelets (thrombocytopenia), which can increase the likelihood of having infections or bleeding.
- liver disorders (hepatitis) characterized by symptoms including jaundice (yellowing of the skin and/or eyes) and/or pale stools.
- kidney disorders of an inflammatory nature (nephritis) that include the following signs and symptoms: blood in the urine, edema (swelling due to increased fluids), and increased blood pressure
- peripheral neuropathy (a condition that affects the nerves of the hands and feet, including symptoms of tingling and numbness)
- pulmonary and fibrotic allergic reactions (symptoms include cough, difficulty breathing, bronchospasm, bloody or excessive sputum)
- hair loss (this is reversible)
- muscle or joint pain
- inflammation that can affect several parts of the body, such as joints, skin, kidneys, heart, etc. (symptoms include painful joints, fatigue, fever, unexplained bleeding, bruising, purple discoloration of the skin, spots under the skin, including severe skin reactions and severe burns that can affect the skin as a protective barrier of the body)
- semen with low sperm concentration (oligospermia) (this is reversible)
- occasionally, allergic reactions and fever may occur
Frequency not known(cannot be estimated from the available data)
- kidney stones and associated kidney pain (see also section 2)
- change in urine color
- If you experience severe or recurrent headache, vision changes, or ringing or buzzing in the ears. These could be symptoms of increased pressure inside your skull (idiopathic intracranial hypertension).
Some of these adverse reactions may also be attributed to the disease itself.
If these symptoms continue or become more severe, consult your doctor.
Reporting of side effects:
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Medicines and Healthcare Products Agency (AEMPS) website: http://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Pentasa rectal suspension
Keep out of the sight and reach of children.
Do not store above 25°C. Do not refrigerate or freeze. Store in the original container to protect from light.
Do not use this medicine after the expiry date which is stated on the container after “EXP”. The expiry date is the last day of the month shown.
Medicines should not be disposed of via wastewater or household waste. Dispose of the container and any unused medicine in the pharmacy's SIGRE collection point. If you are unsure, ask your pharmacist how to dispose of the container and any unused medicine. This will help protect the environment.
6. Contents of the pack and further information
Composition of Pentasa rectal suspension:
- The active substance is mesalazine.
- The other ingredients are: disodium edetate, sodium metabisulfite, sodium acetate trihydrate, hydrochloric acid, and purified water c.s.p. 100 milliliters.
Appearance and packaging of the product
Pentasa rectal suspension is a white to slightly yellow suspension of 100 milliliters for rectal administration.
Each box contains 7 sachets of rectal suspension of 100 milliliters.
Marketing authorization holder and manufacturer
Marketing authorization holder
Ferring S.A.U
C/ del Arquitecto Sánchez Arcas nº3, 1º
28040 Madrid
Spain
Manufacturer:
Ferring Léciva a.s
Address: K Rybníku 475, 252 42
Jesenice, Prague, Czech Republic
Date of last revision of this leaflet: January 2025
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Healthcare Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price43.52 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to PENTASA 4 g RECTAL SUSPENSIONDosage form: SUPPOSITORY, 1 gActive substance: mesalazineManufacturer: Tillotts Pharma Spain S.L.U.Prescription requiredDosage form: TABLET, 1600 mgActive substance: mesalazineManufacturer: Tillotts Pharma Spain S.L.U.Prescription requiredDosage form: TABLET, 400 mgActive substance: mesalazineManufacturer: Tillotts Pharma Spain S.L.U.Prescription required
Online doctors for PENTASA 4 g RECTAL SUSPENSION
Discuss questions about PENTASA 4 g RECTAL SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions