
PAGRENTIL RETARD 150 mg PROLONGED-RELEASE TABLETS

How to use PAGRENTIL RETARD 150 mg PROLONGED-RELEASE TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Pagrentil Retard 25 mg prolonged-release tablets EFG
Pagrentil Retard 50 mg prolonged-release tablets EFG
Pagrentil Retard 100 mg prolonged-release tablets EFG
Pagrentil Retard 150 mg prolonged-release tablets EFG
Pagrentil Retard 200 mg prolonged-release tablets EFG
Pagrentil Retard 250 mg prolonged-release tablets EFG
tapentadol
Read the entire package leaflet carefully before starting to take this medication, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
If you have any questions, consult your doctor or pharmacist.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What is Pagrentil Retard and what is it used for
- What you need to know before taking Pagrentil Retard
- How to take Pagrentil Retard
- Possible side effects
- Storage of Pagrentil Retard
- Contents of the pack and further information
1. What is Tapentadol Retard Sandoz and what is it used for
Tapentadol - the active ingredient of Pagrentil Retard - is a potent analgesic that belongs to the class of opioids. Pagrentil Retard is used for the treatment of severe chronic pain in adults that can only be adequately treated with an opioid analgesic.
2. What you need to know before taking Tapentadol Retard Sandoz
Do not take Pagrentil Retard:
- if you are allergic to tapentadol or any of the other ingredients of this medication (listed in section 6),
- if you have asthma or if your breathing is slow or shallow to dangerous levels (respiratory depression, hypercapnia),
- if you have intestinal paralysis,
- if you have consumed alcohol, sleeping pills, other analgesics, or other psychotropic medications (medications that affect mood and emotions) at high doses (see section "Other medications and Pagrentil Retard").
Warnings and precautions
Consult your doctor or pharmacist before starting to take tapentadol:
- if your breathing is slow or shallow,
- if you have increased intracranial pressure or altered consciousness up to coma,
- if you have had a head injury or brain tumors,
- if you have liver or kidney disease (see section "How to take Pagrentil Retard"),
- if you have a disease of the pancreas or bile ducts, including pancreatitis,
- if you are taking medications called mixed opioid agonist/antagonists (e.g., pentazocine, nalbuphine) or partial agonists of opioid receptors μ (e.g., buprenorphine),
- if you are prone to epilepsy or convulsive seizures, or if you are taking other medications with a known risk of increasing seizures, as the risk of these seizures may increase,
- if you or a family member have a history of abuse or dependence on alcohol, prescription medications, or illegal substances ("addiction"),
- if you are a smoker,
- if you have ever had problems with your mood (depression, anxiety, or personality disorder) or have received psychiatric treatment for other mental illnesses.
This medication contains tapentadol, an opioid medication. Repeated use of opioid analgesics can reduce their effectiveness (you may get used to it). It can also lead to dependence and abuse, which can result in a potentially fatal overdose. It is essential that you inform your doctor if you think you may have developed dependence on tapentadol. Its use (even at therapeutic doses) can cause physical dependence, which may cause you to experience withdrawal symptoms and a recurrence of your problems if you stop taking this medication abruptly.
Tapentadol can cause physical and psychological addiction. If you have a tendency to abuse medications or are dependent on medications, you should only take these tablets for short periods under strict medical supervision.
Respiratory disorders related to sleep
Tapentadol can cause respiratory disorders related to sleep, such as sleep apnea (pauses in breathing during sleep) and sleep-related hypoxemia (low oxygen levels in the blood). Symptoms may include pauses in breathing during sleep, nighttime awakenings due to difficulty breathing, difficulty maintaining sleep, or excessive daytime sleepiness. If you or someone else observes these symptoms, consult your doctor. Your doctor may consider reducing the dose.
Other medications and Pagrentil Retard
Inform your doctor or pharmacist if you are taking, have recently taken, or may need to take any other medication.
- The risk of side effects increases if you are taking medications that can cause seizures (attacks), such as certain antidepressants or antipsychotics. The risk of seizures increases if you take tapentadol simultaneously with these medications. Your doctor will tell you if tapentadol is suitable for you.
- The concomitant use of tapentadol and sedative medications such as benzodiazepines or related medications (certain sleeping pills or tranquilizers, e.g., barbiturates) or analgesics such as opioids, morphine, and codeine (also as a cough medication), antipsychotics, antihistamines-H1, alcohol, increases the risk of drowsiness, respiratory difficulties (respiratory depression), coma, and can be life-threatening. Due to this, concomitant use should only be considered when other treatment options are not possible.
However, if your doctor prescribes tapentadol with sedative medications, you should limit the dose and duration of concomitant treatment.
The concomitant use of opioids and medications used to treat epilepsy, neuropathic pain, or anxiety (gabapentin and pregabalin) increases the risk of opioid overdose, respiratory depression, and can be potentially fatal.
Inform your doctor if you are taking gabapentin or pregabalin, or any other sedative medication, and strictly follow your doctor's dosage recommendation. It may be helpful to inform your friends and family about the signs and symptoms indicated above. Inform your doctor if you experience any of these symptoms.
- If you are taking a type of medication that affects serotonin levels (e.g., certain medications for treating depression), talk to your doctor before taking tapentadol, as there have been cases of "serotonin syndrome". Serotonin syndrome is a rare but potentially life-threatening disorder. Symptoms include involuntary rhythmic muscle contractions, including muscles that control eye movement, agitation, excessive sweating, tremors, exaggerated reflexes, increased muscle tension, and body temperature above 38°C. Your doctor can provide you with additional information.
- The concomitant administration of tapentadol with other types of medications called mixed opioid agonist/antagonists (e.g., pentazocine, nalbuphine) or partial agonists of opioid receptors μ (e.g., buprenorphine) has not been studied. It is possible that tapentadol may not have the same effectiveness if administered with one of these medications. Inform your doctor if you are currently being treated with one of these medications.
- The administration of tapentadol with potent inhibitors or inducers (e.g., rifampicin, phenobarbital, St. John's Wort) of certain enzymes necessary to eliminate tapentadol from your body may affect the effectiveness of tapentadol or cause side effects, especially when starting or stopping this other type of medication. Keep your doctor informed about all medications you are taking.
- Tapentadol should not be taken with MAO inhibitors (a type of medication for treating depression). Inform your doctor if you are taking MAO inhibitors or if you have taken them in the last 14 days.
Taking Pagrentil Retard with food, drinks, and alcohol
Do not consume alcohol while taking tapentadol, as some of its side effects, such as drowsiness, may increase. Food does not affect the effect of this medication.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
Do not take this medication:
- if you are pregnant, unless your doctor has indicated it. If used during prolonged periods during pregnancy, tapentadol may cause withdrawal symptoms in the newborn, which can be life-threatening if not detected and treated by a doctor.
- during breastfeeding, as it can be excreted in breast milk.
The use of tapentadol is not recommended
- during labor, as it may cause slow or shallow breathing to dangerous levels (respiratory depression) in the newborn.
Driving and using machines
Tapentadol can cause drowsiness, dizziness, and blurred vision, and may affect your reaction ability. This may occur especially at the start of treatment with tapentadol, after a dose change, or when drinking alcohol or taking tranquilizers. Ask your doctor if you can drive or use machines during treatment with tapentadol.
3. How to take Tapentadol Retard Sandoz
Follow the instructions for administration of this medication indicated by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again.
Your doctor will adjust the dose based on the intensity of your pain and your personal level of sensitivity to pain. The minimum effective dose should generally be taken to relieve pain.
Adults
The recommended initial dose is 50 mg twice a day, approximately every 12 hours.
Total daily doses of tapentadol above 500 mg are not recommended.
Your doctor may prescribe a different dose or dosing regimen, and more suitable if necessary. If you think the effect of these tablets is too strong or too weak, consult your doctor or pharmacist.
Elderly patients
In elderly patients (over 65 years of age), the dose does not usually need to be adjusted. However, the elimination of tapentadol may be delayed in certain patients in this age group. If this happens to you, your doctor may prescribe a different dosing regimen.
Liver and kidney diseases (hepatic and renal insufficiency)
Patients with severe liver problems should not take these tablets. If you have moderate liver problems, your doctor will prescribe a different dosing regimen. If you have mild liver problems, the dose does not need to be adjusted.
Patients with severe kidney problems should not take these tablets. If you have mild or moderate kidney problems, the dose does not need to be adjusted.
Use in children and adolescents
Tapentadol is not indicated in children and adolescents under 18 years of age.
How and when to take Pagrentil Retard
Tapentadol should be taken orally.
Always swallow the tablet whole with a sufficient amount of liquid. Do not chew or crush it - this could lead to an overdose because the active ingredient will be released in your body too quickly. You can take the tablets on an empty stomach or with food.
The tablet can be divided into equal doses.
The tablet coating may not be completely digested and may appear in your feces. This should not concern you, as the active ingredient of the tablet will have already been absorbed in your body, and what you see is only the empty coating.
Instructions for opening the blister
This medication is packaged in child-resistant unit dose blisters. You cannot press the tablet through the blister. Note the instructions for opening the blister shown below:
- Tear a single dose along the perforation line of the blister.
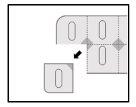
- In the single dose, you can access an unscaled area located in the position where the perforation lines cross.

- Pull the unscaled area to remove the cover.
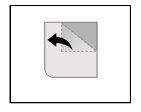
For how long should you take Pagrentil Retard
Do not take the tablets for longer than your doctor has indicated.
If you take more Pagrentil Retard than you should
After taking very high doses, you may experience some of the following effects:
- very small pupils,
- vomiting,
- decreased blood pressure,
- rapid heartbeat,
- fainting, altered consciousness, or coma (deep loss of consciousness),
- seizures,
- slow or shallow breathing to dangerous levels or respiratory arrest.
If you experience any of these effects, contact a doctor immediately.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medication and the amount ingested.
If you forget to take Pagrentil Retard
If you forget to take the tablets, you will likely feel pain again. Do not take a double dose to make up for the missed doses; simply continue taking the tablets as before.
If you stop taking Pagrentil Retard
If you stop or discontinue treatment too early, you will likely feel pain again. If you want to stop treatment, consult your doctor before doing so.
Generally, patients do not experience any side effects after stopping treatment, but in rare cases, people who have taken the tablets for a long time may feel unwell if they stop taking them suddenly.
The symptoms may be:
- restlessness, tearful eyes, runny nose, yawning, sweating, chills, muscle pain, and dilated pupils,
- irritability, anxiety, back pain, joint pain, weakness, abdominal cramps, difficulty sleeping, nausea, loss of appetite, vomiting, diarrhea, and increased blood pressure, respiratory rate, or heart rate.
If you experience any of these symptoms after stopping treatment, consult your doctor.
You should not stop taking this medication abruptly, unless your doctor tells you to do so. If your doctor wants you to stop taking these tablets, they will tell you how to do it, which may involve gradually reducing the dose.
If you have any other questions about the use of this medication, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
Adverse effects or important symptoms to be aware of and what to do if you are affected by them:
- This medicine may cause allergic reactions. The symptoms may consist of wheezing (a kind of whistling when breathing), difficulty breathing, swelling of the eyelids, face or lips, skin rash or itching, especially if they affect the whole body.
- Another serious adverse effect is breathing more slowly or more weakly than normal. It occurs mostly in elderly patients or in weakened patients.
If you experience any of these important symptoms, consult your doctor immediately.
Other adverse effects that may occur:
Very common(may affect more than 1 in 10 people)
- nausea, constipation,
- dizziness, drowsiness, headache.
Common(may affect up to 1 in 10 people)
- decreased appetite, anxiety, depressed mood, difficulty sleeping, nervousness, restlessness, attention disorders,
- tremors, muscle twitches,
- hot flashes,
- shortness of breath,
- vomiting, diarrhea, indigestion,
- itching, increased sweating, skin rashes,
- feeling of weakness, fatigue, feeling of change in body temperature, dryness of the mucous membranes, water retention in the tissues (edema).
Uncommon(may affect up to 1 in 100 people)
- allergic reaction to medicines (including swelling under the skin, hives and in severe cases difficulty breathing, decreased blood pressure, collapse or shock),
- weight loss,
- disorientation, confusion, excitability (agitation), altered perception, sleep disorders, euphoric mood, decreased level of consciousness, memory impairment, mental deterioration,
- fainting, sedation, balance disorders, speech difficulties, numbness, abnormal sensations in the skin (e.g. tingling, itching),
- vision changes,
- rapid heartbeats, slow heartbeats, palpitations, decreased blood pressure,
- abdominal discomfort,
- hives,
- delayed urination, frequent urination,
- sexual dysfunction,
- drug withdrawal syndrome (see section "If you stop treatment with Pagrentil Retard"), feeling of discomfort, irritability.
Rare(may affect up to 1 in 1,000 people)
- drug dependence, altered thinking, epileptic seizures, feeling of being about to faint, altered coordination,
- slow or shallow breathing to dangerous levels (respiratory depression),
- altered gastric emptying,
- feeling of intoxication, feeling of relaxation.
Frequency not known(cannot be estimated from the available data)
- delirium.
In general, the possibility of having suicidal thoughts and behaviors increases in patients with chronic pain. Additionally, some medications for treating depression (with an impact on the brain's neurotransmitter system) may increase this risk, especially at the start of treatment. Although tapentadol also affects neurotransmitters, experience in patients has not proven that it increases this risk.
Reporting of adverse effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that is not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Tapentadol Retard Sandoz
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date that appears on the box and the blister after CAD/EXP. The expiration date is the last day of the month indicated.
This medicine does not require special storage conditions.
Medicines should not be thrown away through the sewers or in the trash. Deposit the containers and the medicines you no longer need in the SIGRE Point of the pharmacy. In case of doubt, ask your pharmacist how to dispose of the containers and medicines you no longer need. This way, you will help protect the environment.
6. Package contents and additional information
Composition of Pagrentil Retard
- The active ingredient is tapentadol.
Pagrentil Retard 25 mg
Each prolonged-release tablet contains tapentadol phosphate equivalent to 25 mg of tapentadol.
Pagrentil Retard 50 mg
Each prolonged-release tablet contains tapentadol phosphate equivalent to 50 mg of tapentadol.
Pagrentil Retard 100 mg
Each prolonged-release tablet contains tapentadol phosphate equivalent to 100 mg of tapentadol.
Pagrentil Retard 150 mg
Each prolonged-release tablet contains tapentadol phosphate equivalent to 150 mg of tapentadol.
Pagrentil Retard 200 mg
Each prolonged-release tablet contains tapentadol phosphate equivalent to 200 mg of tapentadol.
Pagrentil Retard 250 mg
Each prolonged-release tablet contains tapentadol phosphate equivalent to 250 mg of tapentadol.
Pagrentil Retard is available in the following package sizes:
Pagrentil Retard 25 mg
Child-resistant unit-dose blisters of 20, 30, 40, 50, 54, 60 or 100 (clinical package) prolonged-release tablets.
Pagrentil Retard 50 - 250 mg
Child-resistant unit-dose blisters of 20, 24, 30, 50, 54, 60 or 100 (clinical package) prolonged-release tablets.
Not all package sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
Sandoz Farmacéutica, S.A.
Centro Empresarial Parque Norte
Edificio Roble
C/ Serrano Galvache, 56
28033 Madrid
Spain
Manufacturer
Develco Pharma GmbH
Grienmatt 27
DE-79650 Schopfheim
Germany
or
Salutas Pharma GmbH
Otto-von-Guericke-Allee 1,
DE-39179 Barleben
Germany
This medicine is authorized in the Member States of the European Economic Area under the following names:
Germany | Tapentadol - 1 A Pharma 25 mg Retardtabletten Tapentadol - 1 A Pharma 50 mg Retardtabletten Tapentadol - 1 A Pharma 100 mg Retardtabletten Tapentadol - 1 A Pharma 150 mg Retardtabletten Tapentadol - 1 A Pharma 200 mg Retardtabletten Tapentadol - 1 A Pharma 250 mg Retardtabletten |
Slovakia | MABINOVAN 25 mg MABINOVAN 50 mg MABINOVAN 100 mg MABINOVAN 150 mg MABINOVAN 200 mg MABINOVAN 250 mg |
Italy | Tapelod |
Netherlands | Tapentadol Retard Sandoz 25 mg, tabletten met verlengde afgifte Tapentadol Retard Sandoz 50 mg, tabletten met verlengde afgifte Tapentadol Retard Sandoz 100 mg, tabletten met verlengde afgifte Tapentadol Retard Sandoz 150 mg, tabletten met verlengde afgifte Tapentadol Retard Sandoz 200 mg, tabletten met verlengde afgifte Tapentadol Retard Sandoz 250 mg, tabletten met verlengde afgifte |
Czech Republic | Mabinovan |
Date of the last revision of this leaflet:February 2023.
Detailed information about this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price66.03 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to PAGRENTIL RETARD 150 mg PROLONGED-RELEASE TABLETSDosage form: MODIFIED-RELEASE TABLET, 100 mgActive substance: tapentadolManufacturer: Sandoz Farmaceutica S.A.Prescription requiredDosage form: MODIFIED-RELEASE TABLET, 200 mgActive substance: tapentadolManufacturer: Sandoz Farmaceutica S.A.Prescription requiredDosage form: MODIFIED-RELEASE TABLET, 25 mgActive substance: tapentadolManufacturer: Sandoz Farmaceutica S.A.Prescription required
Online doctors for PAGRENTIL RETARD 150 mg PROLONGED-RELEASE TABLETS
Discuss questions about PAGRENTIL RETARD 150 mg PROLONGED-RELEASE TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















