NOVOSEVEN 2 mg (100 KUI) POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE

Cómo usar NOVOSEVEN 2 mg (100 KUI) POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
NovoSeven 1mg (50KUI) polvo y disolvente para solución inyectable
NovoSeven 2mg (100KUI) polvo y disolvente para solución inyectable
NovoSeven 5mg (250KUI) polvo y disolvente para solución inyectable
NovoSeven 8mg (400KUI) polvo y disolvente para solución inyectable
eptacog alfa (activado)
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es NovoSeven y para qué se utiliza
- Qué necesita saber antes de empezar a usar NovoSeven
- Cómo usar NovoSeven
- Posibles efectos adversos
- Conservación de NovoSeven
- Contenido del envase e información adicional
Al dorso: Instrucciones de uso de NovoSeven
1. Qué es NovoSeven y para qué se utiliza
NovoSeven es un factor de coagulación de la sangre. Actúa activando el sistema coagulador de la sangre en el lugar de la hemorragia cuando los factores de coagulación propios del paciente no funcionan.
NovoSeven se utiliza para el tratamiento de hemorragias y la prevención de hemorragias excesivas después de intervenciones quirúrgicas u otros tratamientos importantes. El tratamiento temprano con NovoSeven reduce la cantidad y el tiempo de hemorragia. Funciona en todos los tipos de hemorragias, incluyendo hemorragias articulares. Esto reduce la necesidad de hospitalización y los días de ausencia en el trabajo o en el colegio.
Es usado en ciertos grupos de personas:
- Si tiene hemofilia congénita y no responde de forma normal al tratamiento con los factores VIII o IX
- Si tiene hemofilia adquirida
- Si tiene deficiencia de Factor VII
- Si tiene trombastenia de Glanzmann (un trastorno hemorrágico) y su enfermedad no se puede tratar de forma efectiva mediante transfusión de plaquetas, o si las plaquetas no están fácilmente disponibles.
NovoSeven también se le puede administrar por un médico para tratar un sangrado abundante después del parto, incluso si no tiene un trastorno hemorrágico.
2. Qué necesita saber antes de empezar a usar NovoSeven
No use NovoSeven
- Si es alérgico al eptacog alfa (principio activo de NovoSeven) o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- Si es alérgico a proteínas de ratón, hámster o bovinas (como la leche de vaca).
? Si le ocurre algo de esto, no use NovoSeven. Consulte a su médico.
Advertencias y precauciones
Antes de comenzar el tratamiento con NovoSeven, asegúrese de que su médico sabe:
- Si acaba de someterse a una intervención quirúrgica
- Si acaba de sufrir un traumatismo por aplastamiento
- Si sus arterias son más estrechas debido a una enfermedad (ateroesclerosis)
- Si tiene un mayor riesgo de formación de coágulos sanguíneos (trombosis)
- Si padece una enfermedad hepática grave
- Si padece septicemia grave
- Si es propenso a la coagulación intravascular diseminada (CID, situación en la que se forman coágulos de sangre en el torrente sanguíneo) debe ser monitorizado cuidadosamente.
? Si le ocurre algo de esto, comuníqueselo a su médico antes de usar este producto.
Otros medicamentos y NovoSeven
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
No use NovoSeven al mismo tiempo que concentrados de complejos de protrombinao rFXIII. Debe consultar con su médico antes de usar NovoSeven si también utiliza factor VIII o IX.
Existe una experiencia limitada del uso de NovoSeven junto con otros medicamentos llamados antifibrinolíticos (como ácido aminocaproico o ácido tranexámico) los cuales son utilizados también para el control de las hemorragias. Debe consultar con su médico antes de usar NovoSeven con estos medicamentos.
Embarazo, lactancia y fertilidad
Si está embarazada o en período de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar NovoSeven.
Conducción y uso de máquinas
No hay estudios de los efectos de NovoSeven sobre la capacidad de conducir y usar máquinas. Sin embargo, no hay razón médica para pensar que podría afectar a su capacidad.
NovoSeven contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por inyección, esto es, esencialmente “exento de sodio”.
3. Cómo usar NovoSeven
El polvo de NovoSeven debe ser reconstituido con el disolvente e inyectado vía intravenosa. Vea las instrucciones detalladas al dorso.
Cuándo tratarse
Comenzar el tratamiento de una hemorragia tan pronto como sea posible, lo ideal dentro de las primeras 2 horas.
- En caso de una hemorragia leve o moderada, debe tratarse a sí mismo tan pronto como sea posible, preferentemente en su domicilio.
- En caso de una hemorragia grave debe ponerse en contacto con su médico. Normalmente, las hemorragias graves son tratadas en el hospital y usted puede ponerse la primera dosis de NovoSeven de camino allí.
No debe tratarse durante más de 24 horas sin consultar a su médico.
- Cada vez que use NovoSeven, debe comunicarlo cuanto antes a su médico o a su hospital.
- Si no se controla la hemorragia dentro de las primeras 24 horas, contacte con su médico inmediatamente. Por lo general necesitará atención hospitalaria.
Dosis
La primera dosis debe administrarse lo antes posible tras el inicio de la hemorragia. Consulte a su médico sobre cuándo deben administrarse las inyecciones y cuánto tiempo debe continuar usándolas.
Su médico fijará su dosis, basándose en su peso corporal, estado y tipo de hemorragia.
Para alcanzar el mejor resultado, siga la dosis prescrita atentamente. Su médico puede cambiar la dosis.
Si tiene hemofilia:
La dosis normal es de 90 microgramos por kilogramo de peso corporal; puede repetir la inyección cada 2-3 horas hasta que la hemorragia esté controlada.
Su médico puede recomendarle una única dosis de 270 microgramos por cada kilogramo de peso. No hay experiencia clínica en el uso de esta dosis única en personas mayores de 65 años.
Si tiene deficiencia de Factor VII:
El rango normal de dosis es de 15‑30 microgramos por cada kilogramo de peso corporal, para cada inyección.
Si tiene trombastenia de Glanzmann:
La dosis habitual es de 90 microgramos (rango de 80 a 120 microgramos) por cada kilogramo de peso corporal, para cada inyección.
Si se inyecta más NovoSeven del que debe
Si se inyecta un exceso de NovoSeven, consulte al médico inmediatamente.
Si olvidó ponerse una inyección de NovoSeven
Si olvidó ponerse una inyección de NovoSeven o quiere interrumpir el tratamiento, consulte al médico inmediatamente.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Efectos adversos graves
Raros (pueden afectar hasta 1 de cada 1.000episodios tratados)
- Alergia, hipersensibilidad o reacciones anafilácticas. Los síntomas pueden incluir erupciones cutáneas, picor, enrojecimiento y urticaria; ruidos en el pecho o dificultad para respirar; sensación de mareo o vértigo, e hinchazón intensa de los labios o garganta, o en el lugar de inyección.
- Coágulos sanguíneos en las arterias del corazón (que podrían conducir a un ataque al corazón o angina de pecho), en el cerebro (que podrían llevar a un derrame cerebral) o en el intestino y riñones. Los síntomas pueden incluír dolor intenso en el pecho, dificultad para respirar, confusión, dificultad con el habla o movimiento (parálisis) o dolor abdominal.
Poco frecuentes (pueden afectar hasta 1 de cada 100episodios tratados)
- Coágulos de sangre en las venas de los pulmones, piernas, hígado, riñones o en el lugar de inyección. Los síntomas pueden incluir dificultad en la respiración, hinchazón roja y dolorosa en la pierna y dolor abdominal.
- Falta de eficacia o respuesta disminuida al tratamiento.
? Si aprecia alguno de estos efectos adversos graves, solicite ayuda médica inmediatamente. Explique que ha estado usando NovoSeven.
Recuerde a su médico si tiene un historial previo de reacciones alérgicas ya que puede necesitar un control más exhaustivo. En la gran mayoría de los casos comunicados de trombos, los pacientes tenían predisposición a sufrir trastornos trombóticos.
Otros efectos adversos raros
(pueden afectar hasta 1 de cada 1.000episodios tratados)
- Náuseas (sentirse mareado)
- Dolor de cabeza
- Cambios en algunos resultados de análisis de sangre y de hígado.
Otros efectos adversos poco frecuentes
(pueden afectar hasta 1 de cada 100episodios tratados)
- Erupciones cutáneas, incluyendo sarpullido, picor y urticaria
- Fiebre.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de NovoSeven
- Mantener este medicamento fuera de la vista y del alcance de los niños.
- No utilice este medicamento después de la fecha de caducidad que se indica en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
- Conservar el polvo y el disolvente por debajo de 25 °C.
- Conservar el polvo y el disolvente protegido de la luz.
- No congelar.
- Utilice NovoSeven inmediatamente después de haber mezclado el polvo con el disolvente para evitar infecciones. Si no puede utilizarlo inmediatamente, después de haberlo mezclado, debe conservarlo en el vial con el adaptador de vial y con la jeringa todavía puesta, dentro de la nevera entre 2 °C y 8 °C, durante un máximo de 24 horas. No congele la solución reconstituida de NovoSeven y manténgala protegida de la luz. No conserve la solución sin consultar con su médico o enfermero.
- Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de NovoSeven
- El principio activo es factor de coagulación VIIa recombinante (eptacog alfa activado).
- Los demás componentes en el polvo son cloruro sódico, cloruro cálcico dihidrato, glicilglicina, polisorbato 80, manitol, sacarosa, metionina, ácido clorhídrico, hidróxido sódico. Los componentes del disolvente son histidina, ácido clorhídrico, hidróxido sódico y agua para preparaciones inyectables.
El polvo para solución inyectable contiene: 1 mg/vial (que corresponde a 50 KUI/vial), 2 mg/vial (que corresponde a 100 KUI/vial), 5 mg/vial (que corresponde a 250 KUI/vial) o 8 mg/vial (que corresponde a 400 KUI/vial).
Después de la reconstitución, 1 ml de la solución contiene 1 mg de eptacog alfa (activado).
1 KUI es igual a 1.000 UI (Unidades Internacionales).
Aspecto de NovoSeven y contenido del envase
El vial de polvo contiene polvo blanco y la jeringa precargada contiene una solución transparente e incolora. La solución reconstituida es incolora. No utilice la solución reconstituida si aparecen partículas o si está decolorada.
Cada envase de NovoSeven contiene:
- 1 vial con polvo blanco para solución inyectable
- 1 adaptador para el vial
- 1 jeringa precargada con disolvente para la reconstitución
- 1 émbolo
Presentaciones: 1 mg (50 KUI), 2 mg (100 KUI), 5 mg (250 KUI) y 8 mg (400 KUI).
La presentación actual se indica en el embalaje exterior.
Titular de la autorización de comercialización y responsable de la fabricación
Novo Nordisk A/S
Novo Allé
DK‑2880 Bagsværd, Dinamarca
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
Instrucciones de uso de NovoSeven LEA ESTAS INSTRUCCIONES DETENIDAMENTE ANTES DE USAR NOVOSEVEN NovoSeven se presenta en polvo. Antes de la inyección (administración) se debe reconstituir con el disolvente que le acompaña en la jeringa. El disolvente es una solución de histidina. La reconstitución de NovoSeven se debe inyectar en una vena (inyección intravenosa). El equipo en este envase está diseñado para reconstituir e inyectar NovoSeven. Necesitará también un equipo de administración (catéter y aguja mariposa, toallitas estériles impregnadas en alcohol, vendas de gasa y tiritas). Estos materiales no se incluyen en el envase de NovoSeven. No utilice el equipo sin la formación adecuada por parte de su médico o enfermero. Lávese siempre las manos y asegúrese de que la zona a su alrededor esté limpia. Cuando prepara e inyecta un medicamento directamente en una vena es importante utilizar una técnica limpia y libre de gérmenes (aséptica).Una técnica inapropiada puede introducir gérmenes capaces de infectar la sangre. No abra el equipo hasta que esté listo para utilizarlo. No utilice el equipo si se le ha caído o si está dañado.En ese caso utilice un nuevo envase. No utilice el equipo si ha caducado.En ese caso utilice un nuevo envase.La fecha de caducidad está impresa después de CAD en la caja de cartón, en el vial, en el adaptador del vial y en la jeringa precargada. No utilice el equipo si sospecha que está contaminado.En ese caso utilice un nuevo envase. No deseche ninguno de los materiales hasta que se haya inyectado la solución reconstituida. El equipo es para un solo uso. | |
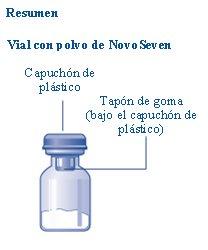
Contenido El envase contiene:
| |
| |
|
|
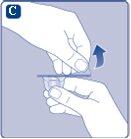
Si el capuchón de plástico está suelto o falta, no use el vial.
|
|
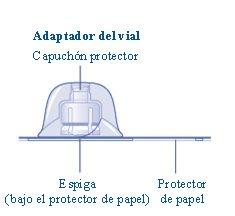
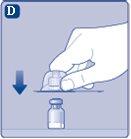
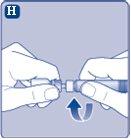
Si el precinto de papel no está completamente sellado o si está roto, no utilice el adaptador del vial. No saque el adaptador del vial del capuchón protector con los dedos.Si toca la espiga del adaptador del vial puede transferirle gérmenes de sus dedos. |
|
Una vez unidos, no retire el adaptador del vial del vial. |
|
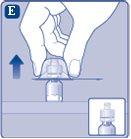
Retire el capuchón protectordel adaptador del vial. No quite el adaptador del vial del vialcuando retire el capuchón protector. |
|
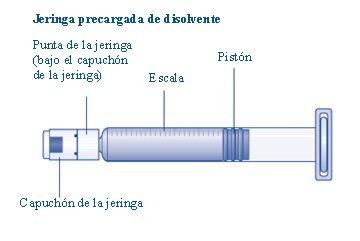
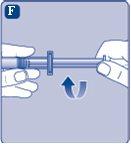
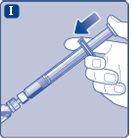
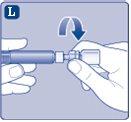
Conecte inmediatamenteel émbolo con la jeringa girándolo en el sentido de las agujas del reloj en el pistón dentro de la jeringa precargada hasta que note resistencia. |
|
No toque la punta de la jeringa bajo el capuchón de jeringa.Si toca la punta de la jeringa, le puede transferir gérmenes de sus dedos. Si el capuchón de la jeringa está suelto o falta, no utilice la jeringa precargada. |
|
|
|
|
|
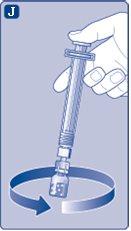
No agite el vial, ya que esto puede producir espuma.
|
|
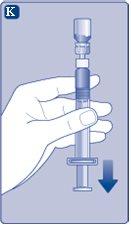
Use el NovoSeven reconstituido inmediatamentepara evitar infecciones. Si no puede usarlo inmediatamente,ver sección 5Conservación de NovoSevenen la otra cara de este prospecto. No conserve la solución reconstituida sin consultarlo con su médico o enfermero. (I) Si su dosis requiere más de un vial, repita los pasos de la Aa la Jcon viales, adaptadores de vial y jeringas precargadas adicionales, hasta que alcance la dosis requerida. | |
|
|
|
|
Injección de NovoSeven con jeringa precargada a través de conectores sin aguja para catéteres intravenosos (IV) Precaución:La jeringa precargada es de cristal y está diseñada para ser compatible con conexiones luer-lock estándar. Algunos conectores sin aguja que presentan una espiga interna son incompatibles con la jeringa precargada. Esta incompatibilidad puede impedir la administración del medicamento y/o originar un daño del conector sin aguja. Siga las instrucciones de uso del conector sin aguja. La administración mediante un conector sin aguja puede requerir extraer la solución reconstituida con una jeringa de plástico estéril luer-lock estándar de 10 ml. Esto debe llevarse a cabo justo después del paso J. | |
NovoSeven está ahora listo para inyectar en una vena.
Inyección de la solución a través de un dispositivo de acceso venoso central (DAVC) como un catéter venoso central o un puerto subcutáneo:
| |
Eliminación
|
|
No desmonte el equipo antes de desecharlo. No reutilice el equipo. |
- País de registro
- Disponibilidad en farmacias
Problema de suministro reportado
Los datos de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) indican un problema de suministro que afecta a este medicamento.<br><br>La disponibilidad puede ser limitada en algunas farmacias.<br><br>Para actualizaciones o alternativas, consulte a su farmacéutico. - Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a NOVOSEVEN 2 mg (100 KUI) POLVO Y DISOLVENTE PARA SOLUCION INYECTABLEForma farmacéutica: INYECTABLE, 1 MG (45 kIU)Principio activo: coagulation factor VIIaRequiere recetaForma farmacéutica: INYECTABLE, 2 MG (90 kIU)Principio activo: coagulation factor VIIaRequiere recetaForma farmacéutica: INYECTABLE, 5 MG (225 kIU)Principio activo: coagulation factor VIIaRequiere receta
Médicos online para NOVOSEVEN 2 mg (100 KUI) POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de NOVOSEVEN 2 mg (100 KUI) POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes