
CEVENFACTA 1 mg (45 KIU) POWDER AND SOLVENT FOR INJECTABLE SOLUTION

How to use CEVENFACTA 1 mg (45 KIU) POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
CEVENFACTA 1 mg (45 kIU), powder and solvent for solution for injection
CEVENFACTA 2 mg (90 kIU), powder and solvent for solution for injection
CEVENFACTA 5 mg (225 kIU), powder and solvent for solution for injection
eptacog beta (activated)
This medicine is subject to additional monitoring, which will allow for the rapid identification of new safety information. You can help by reporting any side effects you may have. The last section of section 4 will tell you how to report side effects.
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What CEVENFACTA is and what it is used for
- What you need to know before you use CEVENFACTA
- How to use CEVENFACTA
- Possible side effects
- Storage of CEVENFACTA
- Contents of the pack and further information
- Instructions for use of CEVENFACTA.
1. What CEVENFACTA is and what it is used for
CEVENFACTA contains the active substance eptacog beta (activated), a recombinant human coagulation factor VIIa (FVIIarh).
CEVENFACTA is used in adults and adolescents (12 years of age or older) who are born with haemophilia A or B and have developed inhibitors (antibodies). It is used for:
- treatment of bleeding episodes,
- management of bleeding during surgery.
How CEVENFACTA works
This medicine works by forming a blood clot at the site of bleeding when the body's own clotting factors are not working.
2. What you need to know before you use CEVENFACTA
Do not use CEVENFACTA
- if you are allergic to eptacog beta (activated) or any of the other ingredients of this medicine (listed in section 6).
- if you are allergic to rabbits or rabbit proteins.
Warnings and precautions
Before starting treatment with CEVENFACTA, tell your doctor:
- If you have a history of atherosclerosis (when arteries are narrowed due to disease), coronary artery disease (heart disease due to narrowing of the blood vessels that supply the heart), cerebrovascular disease (disease of the blood vessels that supply the brain), crush injury, sepsis (severe blood infection), or blood clots.
- If you have heart disease, heart failure, or an abnormal heart rhythm.
- If you have had a pulmonary embolism (in the lungs) or have undergone heart surgery.
- If you have or have had any other medical problems.
Patients with known casein allergy may have a higher risk of hypersensitivity reactions. If signs or symptoms of hypersensitivity occur, treatment should be discontinued and medical attention should be sought immediately. Symptoms may include urticaria (hives), itching, rash, difficulty breathing, swelling around the mouth and throat, chest tightness, wheezing, dizziness, or fainting, and decreased blood pressure.
Although not observed, the following reactions may occur with CEVENFACTA treatment:
- Blood clots in the arteries of the heart (which can cause a heart attack or angina), in the brain (which can cause a stroke), or in the lungs or deep veins. Symptoms may include sweating and pain in the arms, legs, or abdomen, chest pain, difficulty breathing, loss of sensation or movement, and alteration of consciousness or speech.
- Hypersensitivity or anaphylactic reactions. Symptoms may include urticaria (hives), itching, rash, difficulty breathing, swelling around the mouth and throat, chest tightness, wheezing, dizziness, or fainting, and decreased blood pressure.
- Inhibitors (antibodies) that can cause bleeding problems.
If you experience any of these reactions, consult your doctor before continuing to use CEVENFACTA.
It is important to record the batch number of your CEVENFACTA. Each time you use a new pack of CEVENFACTA, note the date and batch number (which appears on the pack, after 'Lot') and keep this information in a safe place.
Adolescents
The warnings and precautions mentioned apply to both adults and adolescents (12 years of age or older).
Other medicines and CEVENFACTA
Tell your doctor if you have recently taken or might take any other medicines.
You should consult your doctor before using CEVENFACTA if
- you are taking or have recently taken another activated factor VII, activated or non-activated prothrombin complex concentrates,
- you are taking or have recently taken factor XIII,
as the combination of these medicines with CEVENFACTA may increase the risk of thromboembolic events (formation of blood clots in the veins).
You should consult your doctor before starting treatment with CEVENFACTA with these medicines.
Pregnancy, breast-feeding, and fertility
If you are pregnant or breast-feeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before taking this medicine.
Driving and using machines
Dizziness may occur after administration of CEVENFACTA. You should avoid driving or using machines while experiencing this symptom.
CEVENFACTA contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per injection, which is essentially 'sodium-free'.
3. How to use CEVENFACTA
Use of this medicine should be initiated and supervised by a doctor experienced in the treatment of haemophilia and/or bleeding disorders.
CEVENFACTA comes as a powder and must be reconstituted with solvent and injected into a vein (intravenous injection). Refer to the instructions for use guide at the end of this leaflet (section 7).
Self-administration
Injection of medicines requires special training. Do not attempt to self-administer unless your doctor or haemophilia treatment centre has taught you how to do it.
Many people with inhibitors learn to self-inject themselves or with the help of a family member. Once informed, you will need additional injection equipment, apart from your CEVENFACTA kit, to properly treat your bleeding episodes at home. Make sure you have all the necessary injection equipment before preparing the medicine for injection. This additional injection equipment will be provided by your healthcare professional (e.g., your pharmacist or haemophilia treatment centre).
CEVENFACTA can be injected at a haemophilia treatment centre, in your doctor's office, or at home. It is essential to treat bleeding as soon as possible to control it.
Start treatment of a bleeding episode as soon as possible, ideally within 2 hours.
? In case of mild or moderate bleeding (e.g., joint, superficial muscle, soft tissue, and mucous membranes), you should treat yourself as soon as possible, ideally at home.
? In case of severe bleeding (e.g., limb [arm or leg] bleeding or life-threatening bleeding, intracranial [in the skull] or gastrointestinal [in the stomach or intestine] bleeding), you should contact your doctor.
Severe bleeding is usually treated in the hospital, but the first dose of CEVENFACTA can be administered on the way to the medical facility.
Do not treat yourself for more than 24 hours without consulting your doctor.
? Each time you use this medicine, inform your healthcare professional as soon as possible.
? If bleeding is not controlled within 24 hours, contact your healthcare professional or emergency service immediately. You will usually need hospital assistance.
For reconstitution of the medicine before administration, follow the guide of the Instructions for useat the end of this leaflet (section 7).
Inject the solution into the vein over 2 minutes or less.
Follow your doctor's instructions for administration of this medicine exactly. If in doubt, consult your doctor again.
Dose
Your healthcare professional will tell you how much CEVENFACTA to use and when to administer the medicine based on your weight, condition, and type of bleeding.
Treatment of bleeding episodes
Treatment with this medicine should be started as soon as a bleeding episode occurs.
Mild and moderate bleeding:
Home treatment should not last more than 24 hours in case of mild to moderate bleeding episodes. Continuation of home treatment after 24 hours should only be considered after consultation at a haemophilia treatment centre.
Severe bleeding:
Seek immediate medical attention if signs or symptoms of severe bleeding occur at home.
To avoid any delay in treatment, the first dose can be administered on the way to the haemophilia treatment centre or doctor's office.
Follow the instructions for administration of the medicine contained in this leaflet or as indicated by your doctor. If in doubt, consult your doctor again.
Method of administration
For reconstitution of the medicine before administration and administration instructions, follow the guide of the Instructions for useat the end of this leaflet (section 7).
If you use more CEVENFACTA than you should
If you use too much CEVENFACTA, go to your doctor immediately.
If you forget to use CEVENFACTA:
If you forget to use CEVENFACTA, consult your doctor.
If you have any further questions on the use of this medicine, ask your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Common side effects
(may affect up to 1 in 10 people)
- Dizziness
- Headache
- Discomfort at the injection site
- Bruising at the injection site (haematoma)
- Increased body temperature
- Postoperative haematoma
- Injection-related reaction
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of CEVENFACTA
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and carton after EXP. The expiry date is the last day of the month shown.
Store below 30°C.
Do not freeze.
Keep the vial in the outer packaging to protect it from light.
For reconstitution of CEVENFACTA, use only the material provided in the kit.
After reconstitution, the product should be stored in the vial and administered within 4 hours. Any remaining solution should be discarded 4 hours after reconstitution.
Do not use this medicine if you notice that the liquid contains particles or is cloudy after mixing.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Composition of CEVENFACTA
- The active ingredient is recombinant coagulation factor VIIa (eptacog alfa (activated))
- The other excipients are:
Powder: arginine hydrochloride, isoleucine, sodium citrate dihydrate, glycine, lysine hydrochloride, polysorbate 80, hydrochloric acid (for pH adjustment).
Solvent: water for injectable preparations.
See section 2 "CEVENFACTA contains sodium"
The powder for injectable solution contains: 1 mg/vial (which corresponds to 45 kIU/vial), 2 mg/vial (which corresponds to 90 kIU/vial), 5 mg/vial (which corresponds to 225 kIU/vial).
After reconstitution, the concentration of the solution is approximately 1 mg/ml (45 kIU/ml) of eptacog alfa (activated). 1 kIU is equal to 1,000 IU (International Units).
Appearance of CEVENFACTA and Container Contents
The vial of powder contains lyophilized powder that is white to off-white and the pre-filled syringe of solvent contains a clear and colorless solution. The reconstituted solution should be clear to slightly opaque.
Each package of CEVENFACTA contains:
- 1 glass vial with powder for an injectable solution,
- 1 sterile vial adapter for reconstitution, equipped with a 5 µm filter,
- 1 pre-filled syringe with water for injections,
- 1 plunger rod with a rear stopper.
Presentation: 1 mg (45 kIU), 2 mg (90 kIU), and 5 mg (225 kIU).
Marketing Authorization Holder
Laboratoire français du Fractionnement et des Biotechnologies
Tour W
102 Terrasse Boieldieu, 19th Floor
92800 Puteaux
France
Manufacturer
LFB Biotechnologies
Zone d’activité de Courtaboeuf
3 Avenue des Tropiques
91940 Les Ulis
France
+33 1 69 82 70 10
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information about this medication is available on the European Medicines Agency website: http://www.ema.europa.eu.
On the European Medicines Agency website, you can find this leaflet in all languages of the European Union/European Economic Area.
INSTRUCTIONS FOR USE
READ THESE INSTRUCTIONS CAREFULLY BEFORE STARTING TO USE CEVENFACTA
CEVENFACTA comes in the form of powder. Before injection, it must be prepared (reconstituted) with the solvent supplied in the syringe. The solvent is water for injectable preparations. The reconstituted CEVENFACTA must be injected into a vein (for intravenous use only).
This kit provides the necessary equipment to reconstitute the medication. Additional materials are needed to inject the medication after reconstitution. These materials will be provided by your healthcare professional (e.g., your pharmacist or your hemophilia treatment center).
Your doctor or nurse will show you and/or your caregiver how to prepare and inject CEVENFACTA. Do not use this kit without proper training by your healthcare professional or your hemophilia treatment center.
Use a clean and germ-free (aseptic) technique when preparing and injecting the medication.
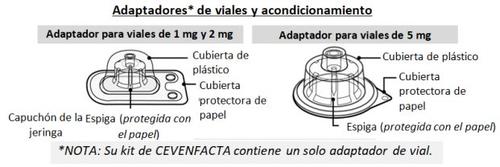
A CEVENFACTA kit contains:
- 1 glass vial with powder for an injectable solution
- 1 sterile vial adapter for reconstitution, equipped with a 5 µm filter
- 1 pre-filled syringe with water for injectable preparations
- 1 plunger rod with a rear stopper

You will also need a sterile injection set (butterfly needle and syringe), a sterile plastic syringe, alcohol-impregnated swabs, and a puncture-proof container for disposing of sharp objects that comply with local regulations and guidelines. These materials are not included in the CEVENFACTA packaging.These materials will be provided by your healthcare professional (e.g., your pharmacist or your hemophilia treatment center).
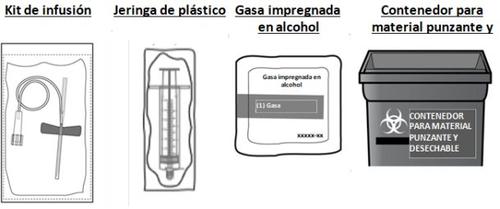
- Gather equipment and prepare the vial
- Take out the number of CEVENFACTA kits you need to administer the prescribed dose, a sterile injection set (not supplied), and an alcohol swab (not supplied).
Do not use the kit if the security seal is broken or if there are signs that the kit is contaminated. Use a new one instead.
- Check the expiration date on the side of the kit (Fig. A).
Do not use it after the expiration date.
- Check the name, concentration, and color of the box to ensure it contains the correct product (the 1 mg packaging is yellow, the 2 mg packaging is green, and the 5 mg packaging is purple).
- Work on a clean and flat surface before starting the steps for reconstituting CEVENFACTA.
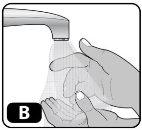
- Wash your hands with soap and water and dry them with a clean towel or let them air dry (Fig. B).
- Take out the contents of a kit and an alcohol swab. Place everything on a clean surface (Fig. C).

- Inspect all the items contained in the kit. Make sure each vial has a syringe of the same color.
Do not use the contents if they have been dropped or are damaged. Use a new kit instead.
- Let the vial and pre-filled syringe reach room temperature if necessary. To do this, hold them until they feel as warm as your hands.
Do not heat the vial and pre-filled syringe in any other way.
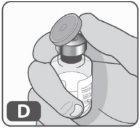
- Remove the plastic cap from the vial (Fig. D).
If the plastic cap is loose or the vial comes without a cap, do not use it.
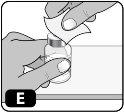
- Clean the rubber stopper with an alcohol swab (Fig. E)and let it air dry for a few seconds to ensure it is as germ-free as possible.
- After cleaning, avoid touching the rubber stopper with your fingers and do not let it come into contact with any other objectbefore placing the vial adapter, as this could contribute to the transmission of germs.
- Place the vial adapter
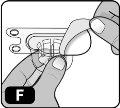
- Remove the paper protective cover from the vial adapter packaging (Fig. F).
If the paper protector is not completely sealed or is broken, do not use the adapter.
Do not remove the vial adapter from its protective packaging with your fingers.If you touch the spike of the adapter with your fingers, you may transmit germs.
- Place the vial on a clean and flat surface and hold it with one hand. With the other hand, place the plastic cover (with the vial adapter inside) directly over the vial and insert the spike of the adapter into the center of the gray rubber stopper.
- Press down firmly so that the spike of the adapter is inserted into the rubber stopper (you may hear and/or see it click into place) (Fig. G).
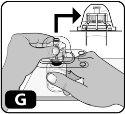
- Remove the plastic cover from the adapter by pressing lightly and lifting it upwards (Fig. H).
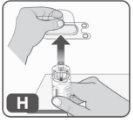
Once you have removed the plastic cover, do not touch the top of the adapter to avoid transmitting germs.
NOTE:The 5 mg vial adapter may not lie flat against the vial, but it is still fully functional. As mentioned earlier, your CEVENFACTA kit contains a single vial adapter (the one suitable for the vial included in the kit).
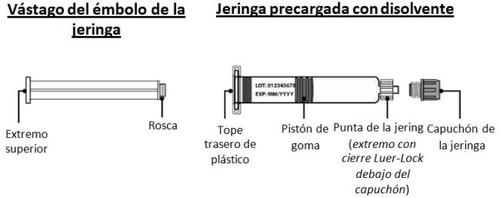
- Place the pre-filled syringe and insert the plunger rod
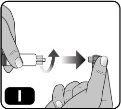
- Remove the cap from the pre-filled syringe by holding the syringe body with one hand and using the other to unscrew it (turning to the left) (Fig. I).
Avoid touching the tip of the syringe to avoid transmitting germs from your fingers.
If the syringe cap is loose or the syringe comes without one, do not use it.
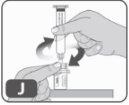
- Hold the edges of the vial adapter and screw the pre-filled syringe by turning it to the right a few times until you feel resistance (Fig. J).
Be careful not to overtighten, as you will need to remove the syringe later.
- To place the plunger rod in the syringe, hold the top end of the plunger rod with one hand and the syringe body with the other.
- Insert the plunger rod into the syringe and then screw it with a few turns (turning to the right) to secure it to the gray rubber piston of the syringe (Fig. K).
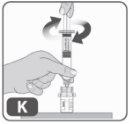
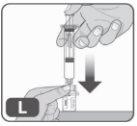
- Mix the medication in the vial
- Slowly push the plunger rod all the way down to the bottom of the syringe to inject all the liquid from the syringe into the vial (Fig. L).
Do not push too quickly, as this may cause excessive foam and air to form in the vial.
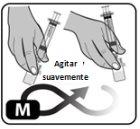
- Gently rotate the vial or roll it carefully between your hands to dissolve all the powder (Fig. M).
Do not shake the vial, as this may cause foam and air to form.
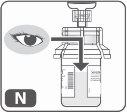
- Visually inspect the final solution (Fig. N)to ensure it is clear or slightly opaque. All the powder should be dissolved, and there should be no particles floating in the liquid.
Do not use the product if the solution contains any particles or is cloudy after mixing.Repeat the process with a new kit.
- Remove the empty syringe from the vial adapter
- Without putting any medication back into the syringe, unscrew it from the vial adapter (turning to the left) until it is completely removed (Fig. O).
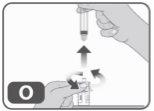
- Dispose of the empty syringe in an authorized puncture-proof container (Fig. P).
Do not remove the vial adapter.
Do not touch the top of the adapter with the Luer-Lock closure. If you touch it, you may transmit germs from your fingers.

- Mix the vial(s) and inject the dose
- If your dose requires more than one vial, repeat the previous steps with additional kits until you have the required dose.
- Withdraw the liquid medication from the vial(s) using a sterile syringe provided by your pharmacy that is large enough to hold the prescribed dose.
- CEVENFACTA must be administered within 4 hours after reconstitution (Fig. Q).
Do not use if more than 4 hours have passed since reconstitution.

- CEVENFACTA can be used for intravenous injection of 2 minutes or less in a vein, as directed by your healthcare professional.
- Dispose of empty medication vials
- After reconstitution and injection, safely dispose of the vial with its adapter, the injection syringe, and any other disposable material in an authorized puncture-proof container (Fig. R).
Do not throw away in household trash.
Do not separate the vial from the vial adapter before disposing of them.
Do not reuse any of the items contained in the kit.

Follow local regulations and guidelines for the proper disposal of the puncture-proof container.
Storage
CEVENFACTA comes in a kit that should be stored below 30°C.
Do not open the items in the kit until you are ready to use them.
Do not freeze or store in syringes that contain the reconstituted CEVENFACTA solution.
Avoid exposing the reconstituted CEVENFACTA solution to direct light.
Important Information
CEVENFACTA is only injected into a vein (intravenous administration). Do not inject it by any other route, such as under the skin (subcutaneous) or into a muscle (intramuscular). Contact your doctor, nurse, or pharmacist if you have any problems.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CEVENFACTA 1 mg (45 KIU) POWDER AND SOLVENT FOR INJECTABLE SOLUTIONDosage form: INJECTABLE, 2 mg (90 kIU)Active substance: coagulation factor VIIaPrescription requiredDosage form: INJECTABLE, 5 mg (225 kIU)Active substance: coagulation factor VIIaPrescription requiredDosage form: INJECTABLE, 1 mg (50 IU)Active substance: coagulation factor VIIaManufacturer: Novo Nordisk A/SPrescription required
Online doctors for CEVENFACTA 1 mg (45 KIU) POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Discuss questions about CEVENFACTA 1 mg (45 KIU) POWDER AND SOLVENT FOR INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















