
MULTIBIC 3 mmol/L POTASSIUM SOLUTION FOR HEMODIALYSIS AND HEMOFILTRATION


How to use MULTIBIC 3 mmol/L POTASSIUM SOLUTION FOR HEMODIALYSIS AND HEMOFILTRATION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
multiBic 3 mmol/l potassium solution for haemodialysis and haemofiltration
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or nurse.
- If you experience any side effects, talk to your doctor or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the package leaflet
- What is multiBic 3 mmol/l potassium and what is it used for
- What you need to know before you start using multiBic 3 mmol/l potassium
- How to use multiBic 3 mmol/l potassium
- Possible side effects
- Storage of multiBic 3 mmol/l potassium
Contents of the pack and further information
1. What is multiBic 3 mmol/l potassium and what is it used for
multiBic 3 mmol/l potassium is a continuous renal replacement therapy solution for the elimination of waste products from the body in people with kidney disease. It is used in patients with kidney injury and also for the treatment of intoxications. The type of solution administered depends on the amount of potassium (a salt) in the blood. Your doctor will regularly check your potassium levels.
2. What you need to know before you start using multiBic 3 mmol/l potassium
Do not use multiBic 3 mmol/l potassium if
- you are allergic to the active substances or to any of the other components of this medicine (listed in section 6).
- you have hypokalaemia (your potassium levels are too low)
- you have metabolic alkalosis (when you have too much bicarbonate in the blood)
- you cannot achieve sufficient blood flow through the haemofilter (filter used in blood filtration).
- you have a high risk of bleeding related to the medications needed to prevent coagulation in the haemofilter.
Warnings and precautions
Talk to your doctor before you start using multiBic 3 mmol/l potassium
- Do not use before mixing the two solutions in a double-chamber bag (two compartments)
- Must not be used if the solution temperature is below ambient temperature
- The tubing lines used to administer the ready-to-use solution must be inspected every 30 minutes.
If a precipitate (solid matter) is observed within these tubing lines, the bags and tubing lines must be replaced immediately and the patient must be carefully monitored.
- Your doctor will check your hydration status (amount of water in your body), potassium, sodium, other salts, certain waste products, and blood sugar levels). Your doctor may also advise you on your diet.
Children
The use of multiBic 3 mmol/l potassium has not been established in children.
Using multiBic 3 mmol/l potassium with other medicines
Tell your doctor if you are using, have recently used, or might use any other medicines.
The following interactions may occur:
- Toxic effects of digitalis (medicines for treating heart diseases)
- Electrolyte substitutions, parenteral nutrition (intravenous feeding), and other treatments with infusions. When using this therapy, its effect on serum blood concentration and fluid status must be taken into account.
- This therapy may reduce the blood concentration of medicines. A dose adjustment may be necessary.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine.
There are no data or limited data on the use of multiBic 3 mmol/l potassium in pregnant and breastfeeding women.
This medicine should only be used during pregnancy if your doctor considers the treatment necessary.
Breastfeeding is not recommended during treatment with multiBic 3 mmol/l potassium.
3. How to use multiBic 3 mmol/l potassium
multiBic 3 mmol/l potassium will be administered to you in a hospital or clinic. Your doctor knows how to use this medicine.
If you have any other questions about the use of this medicine, ask your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The side effects of multiBic 3 mmol/l potassium include:
- nausea
- vomiting
- muscle cramps
- changes in blood pressure
Some side effects may be caused by having too much fluid or too little fluid. These are:
- difficulty breathing
- swelling of the ankles and legs
- dehydration (e.g. dizziness, muscle cramps, feeling of thirst)
- blood disorders (e.g. abnormal salt concentrations in the blood)
The exact frequency of these events cannot be estimated from the available data.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. You can also report side effects directly through the Spanish Medicines and Healthcare Products Agency: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of multiBic 3 mmol/l potassium
Keep this medicine out of the sight and reach of children.
Do not store below 4 °C.
Storage conditions after mixing the two compartments:
The ready-to-use solution must not be stored at a temperature above 30 °C and must be used within 48 hours.
Do not use this medicine after the expiry date which is stated on the packaging after EXP. The expiry date is the last day of the month shown.
6. Contents of the pack and further information
Composition of multiBic 3 mmol/l potassium
- The active substances are potassium chloride, sodium chloride, sodium bicarbonate, calcium chloride dihydrate, magnesium chloride hexahydrate, and glucose monohydrate.
- The other components are water for injections, 25% hydrochloric acid, carbon dioxide, and sodium dihydrogen phosphate dihydrate.
Appearance and packaging of the product
It is available in a double-chamber bag (two compartments containing different solutions). Mixing the solutions from the two compartments results in the ready-to-use solution.
Each bag contains 5,000 ml of solution in total. The ready-to-use solution is clear and colourless.
Each bag is equipped with an HF connector, a Luer lock connector, an injection port, and is covered by a protective sheet.
Pack size:
2 bags of 5,000 ml
Marketing authorisation holder and manufacturer
Marketing authorisation holder
Fresenius Medical Care Deutschland GmbH, Else-Kröner-Straße 1, 61352 Bad Homburg v.d.H., Germany
Manufacturer
Fresenius Medical Care Deutschland GmbH, Frankfurter Straße 6-8, 66606 St. Wendel, Germany
Local representative
Fresenius Medical Care España S.A.
C/ Ronda de Poniente, 8, planta baja, Parque Empresarial Euronova,
28760 Tres Cantos (Madrid)
Spain
Date of last revision of this leaflet: March 2025
Detailed and up-to-date information on this medicine is available on the website of the Spanish Agency for Medicines and Healthcare Products (AEMPS) http://www.aemps.gob.es/
Information intended for healthcare professionals only, see the end of this leaflet.
------------------------------------------------------------------------------------------------------------
The following information is intended for healthcare professionals only:
1,000 ml of the ready-to-use solution contains:
Potassium chloride | 0.2237 g |
Sodium chloride | 6.136 g |
Sodium bicarbonate | 2.940 g |
Calcium chloride dihydrate | 0.2205 g |
Magnesium chloride hexahydrate | 0.1017 g |
Glucose monohydrate | 1.100 g |
(Glucose) | (1.000 g) |
K+ | 3.0 mmol/l |
Na+ | 140 mmol/l |
Ca2+ | 1.5 mmol/l |
Mg2+ | 0.50 mmol/l |
Cl- | 112 mmol/l |
HCO3- | 35 mmol/l |
Glucose | 5.55 mmol/l |
pH ≈ 7.4
Theoretical osmolality: 298 mOsm/l
Do not use if the solution is not clear and colourless and if the bag or connectors are damaged.
For single use only. Any unused solution must be discarded.
Use only through a pump integrated into the extracorporeal blood purification device.
Instructions for use
The haemodialysis and haemofiltration solution must be administered in three stages:
- Removal of the overbag and detailed inspection of the bag
The overbag must be removed only immediately before administration.
Plastic containers can occasionally be damaged during transport from the manufacturing site to the clinic or within the clinic itself. In the solution, this can lead to contamination and microbiological or fungal growth. Therefore, a careful visual inspection of the bag and solutions is necessary before use. Particular attention should be paid to even minor damage to the stopper, welds, and edges of the bag.
- Mixing of the two compartments
The two solutions must be mixed immediately before use to obtain the ready-to-use solution.
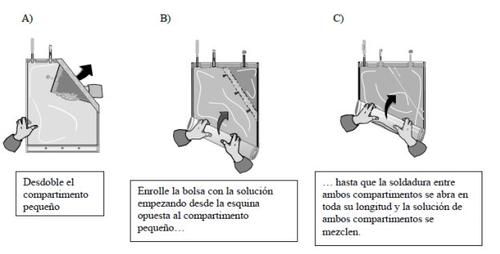
After both compartments have been mixed, it must be verified that the weld is completely open, that the solution is clear and colourless, and that the container does not leak.
- Administration of the ready-to-use solution
The ready-to-use solution must be used immediately, within 48 hours of mixing.
Any addition to the ready-to-use solution must only be made after the ready-to-use solution has been completely mixed. After making an addition, the ready-to-use solution must be thoroughly mixed again before use.
The addition of a sodium chloride solution (up to 30%) or alternatively water for injections is compatible with this medicine and can be used to adjust the sodium concentration if necessary to limit the rate of changes in sodium concentration in case of severe hypernatraemia or hyponatraemia. For more details, see the summary of product characteristics.
If not otherwise prescribed, the ready-to-use solution must be warmed immediately before perfusion to 36.5°C - 38.0°C. The exact temperature must be selected depending on the clinical requirements and the technical equipment used.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to MULTIBIC 3 mmol/L POTASSIUM SOLUTION FOR HEMODIALYSIS AND HEMOFILTRATIONDosage form: HEMOFILTRATION, 2 mmol potassium/lActive substance: HemofiltratesManufacturer: Nikkiso BelgiumPrescription requiredDosage form: HEMOFILTRATION, 4 mmol potassium/lActive substance: HemofiltratesManufacturer: Nikkiso BelgiumPrescription requiredDosage form: HEMOFILTRATION, -Active substance: HemofiltratesManufacturer: Nikkiso BelgiumPrescription required
Online doctors for MULTIBIC 3 mmol/L POTASSIUM SOLUTION FOR HEMODIALYSIS AND HEMOFILTRATION
Discuss questions about MULTIBIC 3 mmol/L POTASSIUM SOLUTION FOR HEMODIALYSIS AND HEMOFILTRATION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















