LEQVIO 284 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE

How to use LEQVIO 284 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Leqvio 284mg solution for injection in pre-filled syringe
Pre-filled syringe with needle protector
will include
Read all of this leaflet carefully before you are given this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the pack
- What is Leqvio and what is it used for
- What you need to know before you are given Leqvio
- How Leqvio is given
- Possible side effects
- Storage of Leqvio
- Contents of the pack and further information
1. What is Leqvio and what is it used for
What is Leqvio and how it works
Leqvio contains the active substance inclisiran. Inclisiran reduces the levels of LDL cholesterol (bad cholesterol), which can cause heart and blood circulation problems when levels are high.
Inclisiran works by interfering with RNA (responsible for transferring genetic information from the body's cells) to limit the production of a protein called PCSK9. This protein can increase LDL cholesterol levels, and by preventing its production, it helps reduce LDL cholesterol levels.
What Leqvio is used for
Leqvio is used in addition to your cholesterol-reducing diet if you are an adult with high cholesterol levels in your blood (primary hypercholesterolemia, including heterozygous familial and non-familial or mixed dyslipidemia).
Leqvio is given:
- in combination with a statin (a type of medicine that treats high cholesterol), sometimes combined with another cholesterol-lowering treatment, if the maximum dose of the statin does not work well enough, or
- alone or in combination with other cholesterol-lowering medicines when statins do not work well or cannot be used.
2. What you need to know before you are given Leqvio
Leqvio must not be given to you
- if you are allergic to inclisiran or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you are given Leqvio:
- if you are receiving dialysis
- if you have severe liver disease
- if you have severe kidney disease
Children and adolescents
Do not use this medicine in children and adolescents under 18 years of age, as there is no experience with the use of this medicine in this age group.
Other medicines and Leqvio
Tell your doctor, pharmacist, or nurse if you are taking, have recently taken, or might take any other medicines.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor, pharmacist, or nurse for advice before using this medicine.
Leqvio should be avoided during pregnancy.
It is not known whether Leqvio passes into breast milk. Your doctor will help you decide whether to continue breastfeeding or start treatment with Leqvio. Your doctor will consider the potential benefits of treatment for you compared to the benefits and risks for your baby's health and breastfeeding.
Driving and using machines
Leqvio is not expected to affect your ability to drive or use machines.
Leqvio contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; it is essentially "sodium-free".
3. How Leqvio is given
The recommended dose of Leqvio is 284 mg administered via a subcutaneous injection (under the skin). The next dose is given 3 months later, and additional doses are given every 6 months.
Before starting Leqvio, you should be following a cholesterol-reducing diet and may be taking a statin. You should maintain the cholesterol-reducing diet and continue taking the statin while receiving Leqvio.
Leqvio is given as a subcutaneous injection into the abdomen; alternative injection sites are the upper arm or thigh. Leqvio will be administered by a doctor, pharmacist, or nurse (healthcare professional).
If you are given too much Leqvio
This medicine will be administered by a doctor, pharmacist, or nurse (healthcare professional). In the unlikely event that too much is given (an overdose), the doctor or other healthcare professional will monitor you for side effects.
If you miss a dose of Leqvio
If you miss an appointment to receive your Leqvio injection, contact your doctor, pharmacist, or nurse as soon as possible to arrange your next injection.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Common(may affect up to 1 in 10 people)
- Injection site reactions, such as pain, redness, or rash.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Leqvio
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and carton after EXP. The expiry date refers to the last day of the month shown.
This medicine does not require any special storage conditions. Do not freeze.
Your doctor, pharmacist, or nurse will check this medicine and dispose of it if it contains particles.
Medicines should not be disposed of via wastewater or household waste. Your doctor, pharmacist, or nurse will dispose of any unused medicine. This will help protect the environment.
6. Container Contents and Additional Information
Leqvio Composition
- The active substance is inclisiran. Each pre-filled syringe contains inclisiran sodium equivalent to 284 mg of inclisiran in 1.5 ml of solution. Each ml contains inclisiran sodium equivalent to 189 mg of inclisiran.
- The other ingredients are water for injections, sodium hydroxide (E524) (see section 2 "Leqvio contains sodium") and phosphoric acid concentrated (E338).
Appearance and Container Contents of the Product
Leqvio 284 mg solution for injection in pre-filled syringe is a clear, colorless to slightly yellowish solution, practically free of particles.
Each pack contains one pre-filled syringe with a needle protector for single use.
Marketing Authorisation Holder
Novartis Europharm Limited
Vista Building
Elm Park, Merrion Road
Dublin 4
Ireland
Manufacturer
Sandoz GmbH
Biochemiestrasse 10
6336 Langkampfen
Austria
Novartis Pharmaceutical Manufacturing GmbH
Biochemiestrasse 10
6336 Langkampfen
Austria
Novartis Pharma GmbH
Roonstrasse 25
90429 Nuremberg
Germany
Novartis Pharma GmbH
Sophie-Germain-Strasse 10
90443 Nürnberg
Germany
You can request more information about this medicinal product from the local representative of the marketing authorisation holder:
Belgium/Belgique/Belgien Novartis Pharma N.V. Tel: +32 2 246 16 11 | Lithuania SIA Novartis Baltics Lietuvos filialas Tel: +370 5 269 16 50 |
Bulgaria Novartis Bulgaria EOOD Tel: +359 2 489 98 28 | Luxembourg/Luxemburg Novartis Pharma N.V. Tel: +32 2 246 16 11 |
Czech Republic Novartis s.r.o. Tel: +420 225 775 111 | Hungary Novartis Hungária Kft. Tel: +36 1 457 65 00 |
Denmark Novartis Healthcare A/S Tel: +45 39 16 84 00 | Malta Novartis Pharma Services Inc. Tel: +356 2122 2872 |
Germany Novartis Pharma GmbH Tel: +49 911 273 0 | Netherlands Novartis Pharma B.V. Tel: +31 88 04 52 111 |
Estonia SIA Novartis Baltics Eesti filiaal Tel: +372 66 30 810 | Norway Novartis Norge AS Tel: +47 23 05 20 00 |
Greece Novartis (Hellas) A.E.B.E. Tel: +30 210 281 17 12 | Austria Novartis Pharma GmbH Tel: +43 1 86 6570 |
Spain Novartis Farmacéutica, S.A. Tel: +34 93 306 42 00 | Poland Novartis Poland Sp. z o.o. Tel: +48 22 375 4888 |
France Novartis Pharma S.A.S. Tel: +33 1 55 47 66 00 | Portugal Novartis Farma – Produtos Farmacêuticos, S.A. Tel: +351 21 000 8600 |
Croatia Novartis Hrvatska d.o.o. Tel: +385 1 6274 220 | Romania Novartis Pharma Services Romania SRL Tel: +40 21 31299 01 |
Ireland Novartis Ireland Limited Tel: +353 1 260 12 55 | Slovenia Novartis Pharma Services Inc. Tel: +386 1 300 75 50 |
Iceland Vistor ehf. Tel: +354 535 7000 | Slovakia Novartis Slovakia s.r.o. Tel: +421 2 5542 5439 |
Italy Novartis Farma S.p.A. Tel: +39 02 96 54 1 | Finland Novartis Finland Oy Tel: +358 (0)10 6133 200 |
Cyprus Novartis Pharma Services Inc. Tel: +357 22 690 690 | Sweden Novartis Sverige AB Tel: +46 8 732 32 00 |
Latvia SIA Novartis Baltics Tel: +371 67 887 070 |
Date of Last Revision of this Prospectus:
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
This information is intended only for healthcare professionals:
Leqvio 284 mg solution for injection in pre-filled syringe
Pre-filled syringe with needle protector
Inclisiran
Healthcare professionals should refer to the Summary of Product Characteristics for full prescribing information.
Indication(see section 4.1 of the Summary of Product Characteristics)
Leqvio is indicated in adults with primary hypercholesterolaemia (heterozygous familial and non-familial) or mixed dyslipidaemia, as an adjunct to diet:
- in combination with a statin or a statin and other lipid-lowering treatments in patients who do not achieve their LDL-C goals with the maximum dose of a statin or,
- alone or in combination with other lipid-lowering treatments in patients who are intolerant to statins, or for whom statins are contraindicated.
Posology (see section 4.2 of the Summary of Product Characteristics).
The recommended dose is 284 mg of inclisiran as a single subcutaneous injection administered at initiation, again at 3 months, and then every 6 months.
Missed Doses
If administration of a planned dose is delayed by less than 3 months, inclisiran should be administered and the patient's original schedule maintained.
If administration of a planned dose is delayed by more than 3 months, a new dosing schedule should be initiated – inclisiran should be administered at initiation, again at 3 months, and then every 6 months.
Transition from Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Inhibitor Monoclonal Antibody Treatment
Inclisiran can be administered immediately after the last dose of a PCSK9 inhibitor monoclonal antibody. To maintain LDL-C reduction, it is recommended to administer inclisiran 2 weeks after the last dose of the PCSK9 inhibitor monoclonal antibody.
Special Populations
Elderly
No dose adjustments are necessary in elderly patients (see section 5.2 of the Summary of Product Characteristics).
Hepatic Impairment
No dose adjustments are necessary in patients with mild (Child-Pugh A) or moderate (Child-Pugh B) hepatic impairment. There are no data available in patients with severe (Child-Pugh C) hepatic impairment (see section 5.2 of the Summary of Product Characteristics). Inclisiran should be used with caution in patients with severe hepatic impairment.
Renal Impairment
No dose adjustments are necessary in patients with mild, moderate, or severe renal impairment or end-stage renal disease (see section 5.2 of the Summary of Product Characteristics). Experience with inclisiran is limited in patients with severe renal impairment. Inclisiran should be used with caution in these patients. See section 4.4 of the Summary of Product Characteristics for precautions in case of haemodialysis.
Paediatric Population
The safety and efficacy of inclisiran in children under 18 years of age have not been established. No data are available.
Method of Administration (see section 4.2 of the Summary of Product Characteristics).
Subcutaneous use.
Inclisiran is administered subcutaneously in the abdomen; alternative injection sites include the upper arm or thigh. Injections should not be made in areas with active skin disease or with wounds such as sunburn, skin rash, inflammation, or skin infections.
Each 284 mg dose is administered via a pre-filled syringe. Each pre-filled syringe is for single use.
Inclisiran is intended for administration by a healthcare professional.
Contraindications (see section 4.3 of the Summary of Product Characteristics).
Hypersensitivity to the active substance or to any of the excipients.
Special Warnings and Precautions for Use (see section 4.4 of the Summary of Product Characteristics).
Haemodialysis
The effect of haemodialysis on the pharmacokinetics of inclisiran has not been studied. Given that inclisiran is eliminated via the kidneys, haemodialysis should not be performed until at least 72 hours after administration of Leqvio.
Storage (see section 6.4 of the Summary of Product Characteristics).
No special storage conditions are required. Do not freeze.
Instructions for Use of Leqvio Pre-filled Syringe with Needle Protector
This section contains information on how to inject Leqvio.
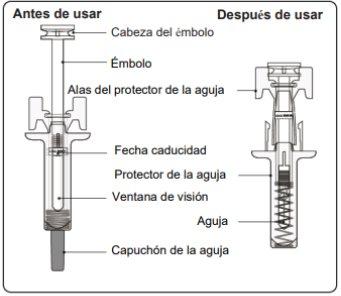
Important Information You Need to Know Before Injecting Leqvio
- Do notuse the pre-filled syringe if any of the seals of the outer packaging or the seal of the plastic tray are broken.
- Do notremove the needle cap until you are ready to inject.
- Do notuse if the pre-filled syringe has been dropped onto a hard surface or dropped after removing the needle cap.
- Do notattempt to reuse or dismantle the pre-filled syringe.
- The pre-filled syringe has a needle protector that will activate to cover the needle when the injection is complete. The needle protector will help prevent needlestick injuries to anyone handling the pre-filled syringe after injection.
Step 1. Inspect the Pre-filled Syringe
You may see air bubbles in the liquid, which is normal. Do notattempt to remove the air.
- Do notuse the pre-filled syringe if it appears damaged or if part of the injectable solution has leaked out of the pre-filled syringe.
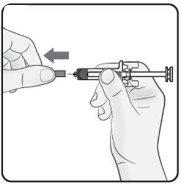
Step 2. Remove the Needle Cap Pull straight and firmly to remove the needle cap from the pre-filled syringe. You may see a drop of liquid at the end of the needle. This is normal. Do notput the needle cap back on. Discard it. Do notremove the needle cap until you are ready to inject. Early removal of the needle cap before injection may cause the finished product to dry out inside the needle, which may lead to blockage. |
|
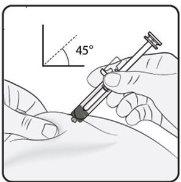
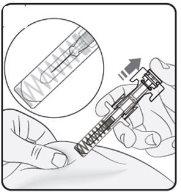
Step 3. Insert the Needle Gently pinch the skin at the injection site and hold the pinch during the injection. With the other hand, insert the needle into the skin at an approximate angle of 45 degrees as shown in the picture. |
|
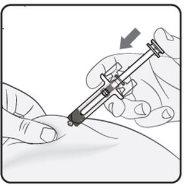
Step 4. Start the Injection Continue to pinch the skin. Press the plunger as far as it will go. This will ensure that the full dose is injected. Note: If you cannot press the plunger after inserting the needle, use a new pre-filled syringe. |
|
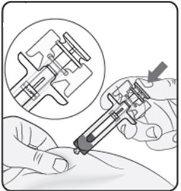
Step 5. Complete the Injection Make sure the plunger head is between the wings of the needle protector as shown in the picture. This will ensure that the needle protector has been activated and will cover the needle once the injection is complete. |
|
Step 6. Release the Plunger While keeping the pre-filled syringe in the injection site, slowly release the plunger until the pre-filled syringe is covered by the needle protector. Remove the pre-filled syringe from the injection site. |
|
Step 7. Dispose of the Pre-filled Syringe Dispose of the pre-filled syringe according to local regulations. |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LEQVIO 284 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGEDosage form: TABLET, 10 mg ezetimibeActive substance: ezetimibeManufacturer: Organon Salud S.L.Prescription requiredDosage form: CAPSULE, 1000 mgActive substance: omega-3-triglycerides incl. other esters and acidsManufacturer: Kern Pharma S.L.Prescription requiredDosage form: CAPSULE, 1000 mgActive substance: omega-3-triglycerides incl. other esters and acidsManufacturer: Strides Pharma (Cyprus) LimitedPrescription required
Online doctors for LEQVIO 284 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE
Discuss questions about LEQVIO 284 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions