
KEVZARA 150 mg SOLUTION FOR INJECTION IN A PRE-FILLED PEN

How to use KEVZARA 150 mg SOLUTION FOR INJECTION IN A PRE-FILLED PEN
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Kevzara 150 mg solution for injection in pre-filled pen
Kevzara 200 mg solution for injection in pre-filled pen
sarilumab
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
In addition to this leaflet, you will be given a patient information card, which contains important safety information that you need before and during treatment with Kevzara.
Contents of the pack
- What is Kevzara and what is it used for
- What you need to know before you use Kevzara
- How to use Kevzara
- Possible side effects
- Storage of Kevzara
- Contents of the pack and other information
1. What is Kevzara and what is it used for
What is Kevzara
Kevzara contains the active substance sarilumab. It is a type of protein called a “monoclonal antibody”.
What is Kevzara used for
Kevzara is used to treat adults with moderate to severe active rheumatoid arthritis (RA) when previous treatments have not worked well or have not been tolerated. Kevzara can be used alone or in combination with a medicine called MTX.
It may help to:
- slow down joint damage
- improve your ability to perform daily activities.
Kevzara is used to treat adults with polymyalgia rheumatica after corticosteroids have been used and have not worked well or if you experience a relapse while tapering the corticosteroid dose (gradual reduction). Kevzara can be used alone or in combination with a medicine called corticosteroid.
How Kevzara works
- Kevzara binds to the receptor of another protein called interleukin-6 (IL-6) and blocks its action.
- IL-6 plays a major role in the symptoms of RA such as pain, joint inflammation, morning stiffness, and fatigue.
2. What you need to know before you use Kevzara
Do not use Kevzara:
- if you are allergic to sarilumab or any of the other ingredients of this medicine (listed in section 6).
- if you have a severe active infection.
Warnings and precautions
Tell your doctor, pharmacist, or nurse if:
- you have any infection or if you get infections often. Kevzara may reduce your body's ability to fight infection, which means you may be more likely to get infections or make your infection worse.
- you have tuberculosis (TB), symptoms of TB (persistent cough, weight loss, lack of interest, mild fever), or have been in close contact with someone with TB. Before starting treatment with Kevzara, your doctor will perform tests for TB.
- you have had viral hepatitis or other liver disease. Before using Kevzara, your doctor will perform a blood test to check your liver function.
- you have had diverticulitis (a disease of the colon) or stomach or intestinal ulcers, or develop symptoms such as fever and stomach pain (abdominal pain) that does not go away.
- you have ever had any type of cancer.
- you have been recently vaccinated or are about to be vaccinated.
If any of the above applies to you (or if you are not sure), talk to your doctor, pharmacist, or nurse before using Kevzara.
Blood tests should be performed before you receive Kevzara. Blood tests should also be performed during your treatment. This is to check if you have a low blood cell count, liver problems, or changes in your cholesterol levels.
Each time you receive a new pack of Kevzara, it is important that you write down the name of the medicine, the date of administration, and the batch number (which appears on the packaging after “Batch”) and keep this information in a safe place.
Children and adolescents
The Kevzara pre-filled pen has not been studied in children from 2 years of age with pJIA and is not indicated for use in children.
Kevzara is not recommended for use in children under 2 years of age. Kevzara should not be administered to children with pJIA who weigh less than 10 kg.
Other medicines and Kevzara
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines. This is because Kevzara may affect the way other medicines work. Other medicines may also affect the way Kevzara works.
In particular, do not use Kevzara and tell your doctor or pharmacist if you are using:
- a group of medicines called “Janus kinase inhibitors (JAK)” (used for diseases such as rheumatoid arthritis and cancer)
- other biologic medicines used to treat RA.
If any of the above applies to you (or if you are not sure), talk to your doctor or pharmacist.
Kevzara may affect the way some medicines work: this means that it may be necessary to change the dose of other medicines. If you are using any of the following medicines, tell your doctor or pharmacist before using Kevzara:
- statins, used to lower cholesterol levels
- oral contraceptives
- theophylline, used to treat asthma
- warfarin, used to prevent blood clots.
If any of the above applies to you (or if you are not sure), talk to your doctor or pharmacist.
Pregnancy and breastfeeding
Talk to your doctor before using Kevzara if you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby.
- Do not use Kevzara if you are pregnant unless your doctor specifically recommends it.
- The effects of Kevzara on the fetus are unknown.
- Your doctor and you must decide if you should receive treatment with Kevzara if you are breastfeeding.
Driving and using machines
Kevzara is not expected to affect your ability to drive or use machines. However, if you feel tired or unwell after receiving treatment with Kevzara, do not drive or use machines.
KEVZARA contains polysorbate 20
This medicine contains 2.28 mg of polysorbate 20 in each 1.14 ml of solution for injection equivalent to 2 mg/ml. Polysorbates may cause allergic reactions. Tell your doctor if you have any known allergies.
3. How to use Kevzara
Treatment should be initiated by a doctor with experience in the diagnosis and treatment of RA or polymyalgia rheumatica. Follow exactly the instructions for administration of this medicine given by your doctor or pharmacist. If you are unsure, talk to your doctor or pharmacist again.
Adult patients
The recommended dose is one 200 mg injection every 2 weeks.
- Your doctor may adjust the dose of your medicine based on the results of your blood tests.
Kevzara is given as an injection under the skin (called a “subcutaneous” injection).
Learn how to use the pre-filled pen
- Your doctor, pharmacist, or nurse will teach you how to inject Kevzara. Following these instructions, Kevzara can be injected by you or administered by a caregiver after receiving adequate training.
- Follow carefully the “Instructions for Use” included in the packaging.
- Use the pre-filled pen exactly as described in the “Instructions for Use”.
If you use more Kevzara than you should
If you have used more Kevzara than you should, talk to your doctor, pharmacist, or nurse.
If you forget to use a dose of Kevzara
If it has been 3 days or less since the missed dose:
- inject your missed dose as soon as possible.
- then give your next dose on the next scheduled day.
If it has been 4 days or more, inject your next dose on the next scheduled day. Do not inject a double dose to make up for the missed injection.
If you are not sure when you should inject your next dose, ask your doctor, pharmacist, or nurse for instructions.
If you stop treatment with Kevzara
Do not stop treatment with Kevzara without talking to your doctor.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Severe side effect
Tell your doctor immediatelyif you think you have an infection(which may affect up to 1 in 10 people). Symptoms may include fever, sweating, or chills.
Adults
Other side effects
Tell your doctor, pharmacist, or nurse if you notice any of the following side effects:
Very common(may affect more than 1 in 10 people):
- low white blood cell count according to blood tests
Common(may affect up to 1 in 10 people):
- infections of the nasal passages or throat, congestion or runny nose, and sore throat (“upper respiratory tract infection”)
- urinary tract infection
- oral herpes
- low platelet count according to blood tests
- high cholesterol, high triglycerides according to blood tests
- abnormal liver function tests
- reactions at the injection site (including redness and itching)
- inflammation of the deep skin tissue
- lung infection
Uncommon(may affect up to 1 in 100 people):
- diverticulitis (a disease that affects the intestine, often with stomach pain, nausea, and vomiting, fever, and constipation, or less frequently diarrhea)
Rare(may affect up to 1 in 1,000 people):
- perforation in the stomach or intestines (a hole that develops in the wall of the intestine)
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is possible that they are not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V*. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Kevzara
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and on the carton after EXP. The expiry date refers to the last day of the month shown.
Store in a refrigerator (between 2 °C and 8 °C).
- Do not freeze.
- Once removed from the refrigerator, do not store Kevzara at a temperature above 25 °C.
- Write the date you removed the pen from the refrigerator on the space provided on the outer packaging.
- Use the pen within 14 days of removing it from the refrigerator or insulated bag.
- Keep the pen in the original packaging to protect from light.
Do not use this medicine if the solution in the pen is cloudy, discolored, or contains particles, or if any part of the pre-filled pen appears damaged.
After use, put the pen in a puncture-resistant container. Keep the container out of the sight and reach of children. Ask your doctor, pharmacist, or nurse how to dispose of the container.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Kevzara Composition
- The active ingredient is sarilumab. Each pre-filled pen contains 150 mg or 200 mg of sarilumab in 1.14 ml of solution.
- The other excipients are arginine, histidine, polysorbate 20 (E 432), sucrose, and water for injectable preparations.
Product Appearance and Container Contents
Kevzara is a clear, colorless to pale yellow injectable solution presented in a pre-filled pen.
Each pre-filled pen contains 1.14 ml of solution providing a single dose. Kevzara is available in packs containing 1 or 2 pre-filled pens and multi-packs containing 3 packs, each with 2 pre-filled pens.
Only some pack sizes may be marketed.
Kevzara is available as 150 mg or 200 mg pre-filled pens.
Marketing Authorization Holder
Sanofi Winthrop Industrie
82 avenue Raspail
94250 Gentilly
France
Manufacturer
Sanofi-Aventis Deutschland GmbH
Brüningstraße 50
Industriepark Höchst
65926 Frankfurt am Main
Germany
Genzyme Ireland Ltd.
IDA Industrial Park
Old Kilmeaden Road
Waterford
Ireland
You can request more information about this medicinal product by contacting the local representative of the marketing authorization holder:
-------------------------------------------------------------------------------------------------------------------
Belgium/Belgique/Belgien Sanofi Belgium Tel: +32 (0)2 710 54 00 | Lithuania Swixx Biopharma UAB Tel: +370 5 236 91 40 |
| Luxembourg/Luxemburg Sanofi Belgium Tel: +32 (0)2 710 54 00 (Belgique/Belgien) |
Czech Republic Sanofi s.r.o. Tel: +420 233 086 111 | Hungary SANOFI-AVENTIS Zrt. Tel.: +36 1 505 0050 |
Denmark Sanofi A/S Tlf: +45 45 16 70 00 | Malta Sanofi S.r.l. +39. 02 39394275 |
Germany Sanofi-Aventis Deutschland GmbH Telephone: 0800 04 36 996 Telephone from abroad: +49 69 305 70 13 | Netherlands Sanofi B.V. Tel: +31 20 245 4000 |
Estonia Swixx Biopharma OÜ Tel: +372 640 10 30 | Norway sanofi-aventis Norge AS Tlf: +47 67 10 71 00 |
Greece Sanofi-Aventis Μονοπρ?σωπη AEBE Τηλ: +30 210 900 16 00 | Austria sanofi-aventis GmbH Tel: +43 1 80 185 – 0 |
Spain sanofi-aventis, S.A. Tel: +34 93 485 94 00 | Poland Sanofi Sp. z o.o. Tel.: +48 22 280 00 00 |
France Sanofi Winthrop Industrie Tél: 0 800 222 555 Appel depuis l’étranger : +33 1 57 63 23 23 | Portugal Sanofi - Produtos Farmacêuticos, Lda Tel: +351 21 35 89 400 |
Croatia Swixx Biopharma d.o.o. Tel: +385 1 2078 500 | Romania Sanofi Romania SRL Tel: +40 (0) 21 317 31 36 |
Ireland sanofi-aventis Ireland Ltd. T/A SANOFI Tel: +353 (0) 1 403 56 00 | Slovenia Swixx Biopharma d.o.o. Tel: +386 1 235 51 00 |
Iceland Vistor ehf. Sími: +354 535 7000 | Slovakia Swixx Biopharma s.r.o. Tel: +421 2 208 33 600 |
Italy Sanofi S.r.l. Tel: 800 536389 | Finland Sanofi Oy Puh/Tel: +358 (0) 201 200 300 |
Cyprus C.A. Papaellinas Ltd. Τηλ: +357 22 741741 | Sweden Sanofi AB Tel: +46 (0)8 634 50 00 |
Latvia Swixx Biopharma SIA Tel: +371 6 616 47 50 |
Date of Last Revision of this Prospectus:
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
-------------------------------------------------------------------------------------------------------------------
Kevzara 150 mg Solution for Injection in Pre-filled Pen
sarilumab
Instructions for Use
The parts of the Kevzara pre-filled pen are shown in this drawing.
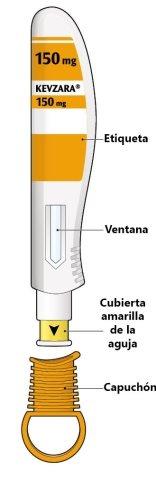
Important Information
This device is a single-dose pre-filled pen (referred to as a “pen” in these instructions). It contains 150 mg of Kevzara for subcutaneous injection once every two weeks.
Ask your healthcare professional to show you how to use the pen correctly before your first injection.
What to Do
- Read all the instructions carefully before using a pen.
- Check that you have the correct medicine and the correct dose.
- Store unused pens in the original container and keep them in the refrigerator at a temperature between 2 °C and 8 °C.
- Keep the container in a thermal bag with a cold pack when traveling.
- Let the pen reach room temperature for at least 60 minutes before using it.
- Use the pen within 14 days of removing it from the refrigerator or thermal bag.
- Keep the pen out of sight and reach of children.
What Not to Do
- Do not use the pen if it has been damaged or if the cap is missing or not attached.
- Do not remove the cap until you are ready for injection.
- Do not press or touch the yellow needle cover with your fingers.
- Do not try to put the cap back on the pen.
- Do not reuse the pen.
- Do not freeze or heat the pen.
- Once removed from the refrigerator, do not store the pen at a temperature above 25 °C.
- Do not expose the pen to direct sunlight.
- Do not inject through clothing.
If you have any further questions, consult your doctor, pharmacist, or nurse.
Step A: Preparation for Injection
- Prepare all the equipment you will need in a clean and flat area.
- You will need an alcohol swab, a cotton ball or gauze, and a sharps container.
- Remove a pen from the container by holding it in the middle of the pen body. Store the remaining pen in the container in the refrigerator.
- Look at the label.
- Check that you have the correct medicine and the correct dose.
- Check the expiration date (EXP), this information is shown on the side of the pens.
- Do notuse the pen if it is expired.
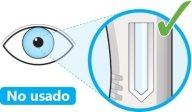
- Look at the window.
- Check that the liquid is clear and colorless to pale yellow.
- You may see an air bubble, this is normal.
- Do notproceed with the injection if the liquid is cloudy, discolored, or contains particles.
- Do notuse the pen if the window is solid yellow.
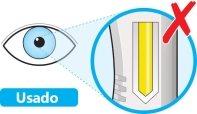
- Place the pen on a flat surface and let it reach room temperature (<25 °C) for at least 60 minutes.
- Using the pen at room temperature may make the injection more comfortable.
- Do notuse the pen if it has been out of the refrigerator for more than 14 days.
- Do notheat the pen; let it warm up naturally.
- Do notexpose the pen to direct sunlight.


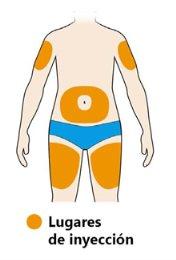
- Select the injection site.
- You can inject into your thigh or stomach (abdomen) except for the 5 cm around your navel. If someone else is giving you the injection, you can also choose the outer upper arm.
- Change the injection site each time you inject.
- Do notinject into sensitive, damaged, or scarred skin.
- Prepare the injection site.
- Wash your hands.
- Disinfect the skin with an alcohol swab.
- Do nottouch the injection site again before the injection.
Step B: Perform the Injection - Proceed with Step B only after completing Step A “Preparation for Injection”
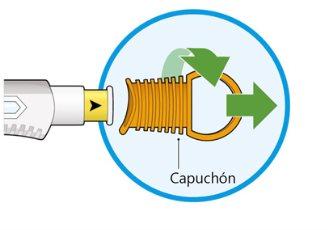
- Twist and remove the orange cap.
- Do notremove the cap until you are ready for the injection.
- Do notpress or touch the yellow needle cover with your fingers.
- Do notput the cap back on.
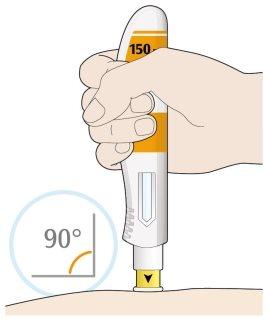
- Place the yellow needle cover on your skin at an angle of approximately 90º.
- Make sure you can see the window.
- Press down and hold the pen firmly against your skin.
- A “click” will be heard when the injection starts.
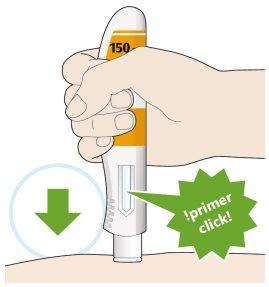
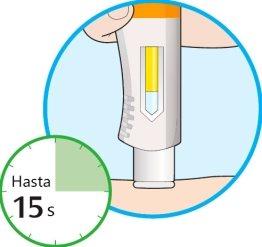
- Continue to hold the pen firmly against your skin.
- The window will start to change color to yellow.
- The injection may take up to 15 seconds (15 s).
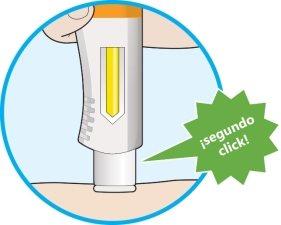
- A second click will be heard. Check that you can see that the entire window has turned completely yellow before removing the pen.
- If you do not hear the second click, continue to check if the window has turned completely yellow.
- If the window has not turned completely yellow, do notadminister a second dose without consulting your healthcare professional.
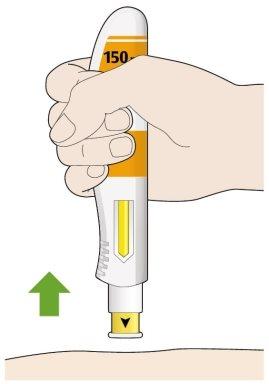
- Remove the pen from your skin.
- If you see a little blood, press on the injection site with a cotton ball or gauze.
- Do notrub the skin after the injection.
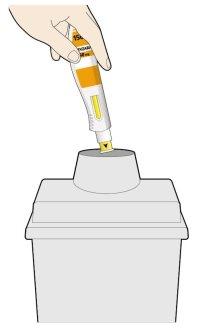
- Put the used pen and cap into a sharps container immediately after use.
- Always keep the container out of sight and reach of children.
- Do notput the cap back on.
- Do notthrow away used pens.
- Do notthrow away the used sharps container unless local regulations permit it. Ask your doctor, pharmacist, or nurse how to dispose of the container.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to KEVZARA 150 mg SOLUTION FOR INJECTION IN A PRE-FILLED PENDosage form: INJECTABLE, 150 mgActive substance: sarilumabManufacturer: Sanofi Winthrop IndustriePrescription requiredDosage form: INJECTABLE, 200 mgActive substance: sarilumabManufacturer: Sanofi Winthrop IndustriePrescription requiredDosage form: INJECTABLE, 200 mgActive substance: sarilumabManufacturer: Sanofi Winthrop IndustriePrescription required
Online doctors for KEVZARA 150 mg SOLUTION FOR INJECTION IN A PRE-FILLED PEN
Discuss questions about KEVZARA 150 mg SOLUTION FOR INJECTION IN A PRE-FILLED PEN, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions







