
HELICOBACTER TEST INFAI 75 mg POWDER FOR ORAL SOLUTION
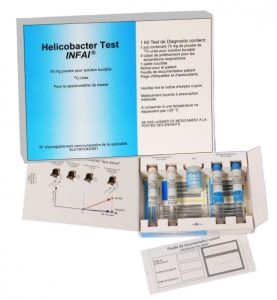

How to use HELICOBACTER TEST INFAI 75 mg POWDER FOR ORAL SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Helicobacter Test INFAI 75 mg powder for oral solution
13C-urea
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What Helicobacter Test INFAI is and what it is used for
- What you need to know before you take Helicobacter Test INFAI
- How to take Helicobacter Test INFAI
- Possible side effects
- Storage of Helicobacter Test INFAI
- Contents of the pack and further information
1. What Helicobacter Test INFAI is and what it is used for
Helicobacter Test INFAI is for diagnostic use only. It is a breath test for adolescents from 12 years of age or adults to determine the presence of a stomach infection caused by the bacteriumHelicobacter pylori.
Why you should take Helicobacter Test INFAI?
You may have a gastric infection caused by the bacterium Helicobacter pylori. Your doctor has recommended that you undergo a Helicobacter INFAI test for one of the following reasons:
- Your doctor wants to confirm whether you have a Helicobacter pyloriinfection in order to diagnose your condition.
- You have already been diagnosed with a Helicobacter pyloriinfection and are being treated to cure it. Now your doctor wants to know if the treatment has been successful.
What does this test consist of?
All foods contain a substance called carbon 13 (13C). This carbon 13 can be detected in the carbon dioxide that is breathed out from the lungs. The actual amount of carbon 13 contained in the breath depends on the type of food that has been ingested.
The test consists of ingesting the "test meal". Then, breath samples are taken. See "Special instructions for use". These samples will be analyzed to measure the "normal" amount of carbon 13 content in the carbon dioxide of your breath.
Then, you will have to drink a solution of carbon 13-urea. Thirty minutes after the administration of the test solution, more breath samples will be taken, whose carbon 13 content will be measured again as described above. The results obtained will be compared, and if a significant increase in carbon 13 content is detected in the second set of samples, this suggests to your doctor the presence of the bacterium Helicobacter pylori.
2. What you need to know before you take Helicobacter Test INFAI
Do not take Helicobacter Test INFAI
- if you have or suspect you have a stomach infectionor a specific inflammation of the stomach lining(atrophic gastritis).
This inflammation of the stomach lining may produce incorrect positive results of the breath test. Additional tests may be required to confirm the presence of Helicobacter pylori.
Warnings and precautions
Consult your doctor or pharmacist before taking Helicobacter Test INFAI if you have any condition that may affect or be affected by the test.
Even if the result of the Helicobacter INFAI test is positive, other tests may be necessary before treatment for Helicobacter pyloriinfection can be started. These are required to check for the presence of any other complications, such as:
- stomach ulcer
- infection of the stomach lining caused by the immune system
- tumors
There is not enough data on the reliability of Helicobacter Test INFAI to recommend its use in patients who have had part of their stomach removed.
If the patient vomits during the test procedure, the test must be repeated. The repetition must be done on an empty stomach and not before the next day.
Using Helicobacter Test INFAI with other medicines
Medicines that affect
- Helicobacter pylori(see section 3, second paragraph in "Method of administration")
- the enzyme urease, which stimulates the reduction of urea
affect Helicobacter Test INFAI.
Tell your doctor or pharmacist if you are taking or have recently taken or may need to take any other medicine.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
It is not expected that taking the breath test during pregnancy or breastfeeding will have a harmful effect.
Driving and using machines
The influence of Helicobacter Test INFAI on the ability to drive and use machines is negligible.
3. How to take Helicobacter Test INFAI
Follow exactly the administration instructions of this medicine indicated by your doctor. In case of doubt, consult your doctor or pharmacist again.
The test must be performed in the presence of your doctor or another qualified person.
The recommended dose is
Patient from 12 years or older should take the content of 1 vial for each test.
Method of administration
The patient must be fasting from 6 hours before the application, preferably during the previous night. Consult your doctor if fasting constitutes a problem, for example, in diabetic patients.
The procedure lasts approximately 40 minutes.
The test must be performed after at least:
- 4 weeks after any treatment for a bacterial infection
- 2 weeks after the last administration of any medicine to reduce acid release in the stomach
Both groups of medicines can influence the results of Helicobacter Test INFAI. Especially after treatment to eradicate Helicobacter pylori. It is important to follow exactly the instructions for use, particularly after a therapy to eradicate Helicobacter, otherwise the result may be questionable.
Essential elements not included in Helicobacter Test INFAI
Before performing the breath test, the test meal must be ingested to delay stomach emptying. This test meal is not included in the package. The following are suitable test meals:
- 200 ml of pure orange juice
- 1 g of citric acid dissolved in 200 ml of water
If there is a reason why you should not take any of these test meals, notify your doctor so that he can suggest an alternative. You will need a glass and water to dissolve the 13C-urea powder. If you need to repeat the test, it will not be repeated before the next day.
Special instructions for use(for mass spectrometry)
This medicine should only be administered by a medical professional and under adequate medical supervision. The patient's data must be documented on the data sheet provided. It is recommended to perform the test at rest.
- The patient must be fasting from 6 hours before the application, preferably during the previous night. If the test is performed later in the day, it is recommended to take only a light meal, perhaps a cup of tea and a toast.
- The test begins with the collection of samples for the determination of baseline values.
- Remove the flexible tube and the two containers with the label: "sampling time: value-minute-00" from the equipment.
- Remove the cap from one of the containers and place the flexible tube without the wrapper inside the container
- Now breathe gently through the tube into the sample container.
- Continue breathing while removing the tube and immediately close the container with its cap. If the sample tube is kept open for more than 30 seconds, the result may be inaccurate.
- Keep the sample container in a vertical position and stick the label, with the barcode, marked "value-minute-00", around the container, so that the barcode is horizontal.
- Now fill the second sample container (labeled: "sampling time: value-minute-00") by breathing in the same way as described before.
- The patient should now drink the test meal (200 ml of pure orange juice or 1 g of citric acid in 200 ml of water)
- Now prepare the test solution in the following way:
- Remove the vial labeled: "13C-urea powder" from the equipment, open it and fill it with tap water up to about three-quarters.
- Close the vial and shake carefully until all the powder is dissolved
- Pour the contents into a glass of water, fill the vial for a second and third time with water and add the contents to the same glass until approximately 30 ml of test solution is obtained.
- The patient should drink this test solution immediately. The time of ingestion should be noted.
- 30 minutes after the administration of the test solution (point 6) the "value-minute-30" samples are collected in both containers that remain in the test package (labeled "sampling time: value-minute-30") as described in points 2 and 3.
Use the labels with the barcode marked "value-minute-30" for these samples.
- The relevant label with the barcode is placed on the patient's data sheet. The four breath sample containers must be returned to the original packaging. This packaging must be sealed with the blue adhesive label.
- The package must be sent for analysis to a qualified laboratory.
Medical professionals or healthcare professionals can find detailed information on breath sample analysis and laboratory test specifications in section 6.6 of the Summary of Product Characteristics.
If you take more Helicobacter Test INFAI than you should
Since only 75 mg of 13C-urea is supplied, an overdose is not expected.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
No side effects are known.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. You can also report side effects directly through the national reporting system listed in Annex V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Helicobacter Test INFAI
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the package after EXP. The expiry date is the last day of the month shown.
Do not store above 25°C.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and further information
Composition of Helicobacter Test INFAI
- The active substance is 13C-urea.
One vial contains 75 mg of 13C-urea.
- It does not contain any other ingredients.
Appearance and package contents
Helicobacter INFAI test is a white and crystalline powder for oral solution.
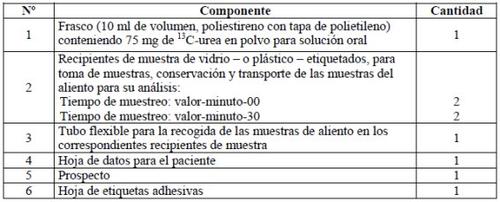
A test kit contains the following parts:
Marketing Authorisation Holder
INFAI GmbH
Riehler Str. 36
D-50668 Köln
Germany
Manufacturer responsible for batch release
INFAI GmbH
An der Kohlenbahn 39
D-58135 Hagen
Germany
You can obtain further information on this medicine from the local representative of the Marketing Authorisation Holder.
AT, BE, BG, CZ, DE, DK, EE, ES, FI, IS, IT, LT, LU, LV, MT, NL, NO, RO, SE
INFAI GmbH, Tel / Tél / Te?. / Τηλ / Puh / Sími / Tlf.: +49 221 880 443
Deutschland / Allemagne / ???????? / Nemecko / Tyskland / Saksamaa / Alemania / Saksa / Þýskaland / Germania / Vokietija / Vacija / Il-Germanja / Duitsland
CY, ELANGELINI PHARMA HELLAS SA, Τηλ: +30 210 626 9200, Ελλ?δα
FRINFAI FRANCE SARL, Tél: +33 389 2043 82
HRPOLIKLINIKA LABPLUS, Tel: + 385 1 299 3595, E-Mail: [email protected]
IE, UKINFAI UK Ltd., Tel: +44 1904 435 228, United Kingdom
PLDr Piktel Medic@l Systems, Tel: +48 85 744 7770
PTSermail Logística Integrada Lda., Tel: +351 21 973 9120
SLPLIVA LJUBLJANA d.o.o., Tel: +386 1 5890 390
HU, SKALLMEDICAL s.r.o, Tel: +421 903 654 103, Szlovákia / Slovenská republika
Date of last revision of this leaflet: {MM/AAAA}.
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to HELICOBACTER TEST INFAI 75 mg POWDER FOR ORAL SOLUTIONDosage form: TABLET, 50 mgActive substance: 13C-ureaManufacturer: Laboratoires Mayoly SpindlerPrescription requiredDosage form: ORAL SOLUTION/SUSPENSION, 45 mgActive substance: 13C-ureaManufacturer: Infai GmbhPrescription requiredDosage form: TABLET, 100 mgActive substance: 13C-ureaManufacturer: Isomed Pharma S.L.Prescription required
Online doctors for HELICOBACTER TEST INFAI 75 mg POWDER FOR ORAL SOLUTION
Discuss questions about HELICOBACTER TEST INFAI 75 mg POWDER FOR ORAL SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















