
DEANXIT FILM-COATED TABLETS

How to use DEANXIT FILM-COATED TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Patient Information Leaflet
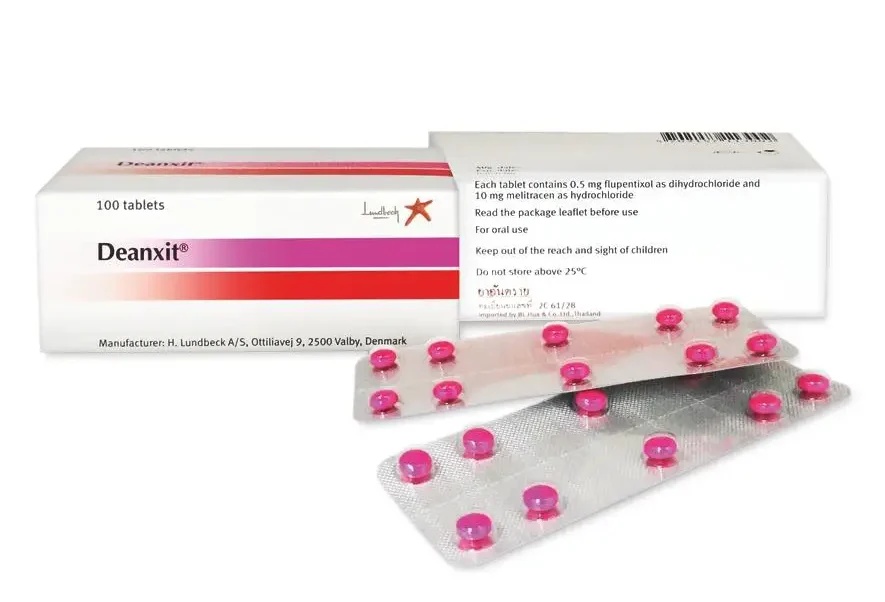
Deanxit film-coated tablets
Flupentixol 0.5 mg (dihydrochloride) and melitracen 10 mg (hydrochloride)
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Deanxit and what is it used for
- What you need to know before you take Deanxit
- How to take Deanxit
- Possible side effects
- Storage of Deanxit
- Contents of the pack and other information
1. What is Deanxit and what is it used for
Deanxit contains the active substances flupentixol and melitracen. Deanxit belongs to a group of medicines that relieve symptoms of depressed mood.
The combination of the active substances forms a preparation with antidepressant, anxiolytic, and activating properties.
Deanxit is used to treat anxiety and depression in patients with or without psychosomatic symptoms.
2. What you need to know before you take Deanxit
Do not take Deanxit:
- If you are allergic to flupentixol, melitracen, or any of the other ingredients of this medicine (listed in section 6).
- If you have reduced consciousness.
- If you have a blood disorder.
- If you have a rare adrenal gland disorder (pheochromocytoma).
- If you have recently had a heart attack (myocardial infarction).
- If you have heart rhythm disturbances that can be seen on an electrocardiogram (ECG).
- If you are taking a medicine known as monoamine oxidase inhibitors (MAOIs) at the same time.
MAOIs include medicines such as phenelzine, iproniazid, isocarboxazid, nialamide, tranylcypromine, and moclobemide, all of which are used to treat depression, and selegiline, which is used to treat Parkinson's disease.
Although you have stopped taking one of the following MAOIs: phenelzine, iproniazid, isocarboxazid, nialamide, or tranylcypromine for depression or selegiline for Parkinson's disease, you should wait 2 weeks before starting to take Deanxit.
You should have waited at least one day since you stopped taking moclobemide.
Deanxit tablets are not suitable for patients with severe depression, such as patients who need to be hospitalized or who require electroconvulsive therapy (ECT), as well as patients who are excitable or hyperactive.
Warnings and precautions
Tell your doctor or pharmacist before you start taking Deanxit if you:
- have an organic brain syndrome (which may be a consequence of alcohol or organic solvent poisoning)
- have a history of seizures
- have difficulty urinating
- have an overactive thyroid gland (hyperthyroidism)
- have a liver or heart disorder
- are more excited or hyperactive than usual, as this medicine may increase this feeling.
- have hypokalemia or hypomagnesemia (too little potassium or magnesium in your blood or a genetic predisposition to either of these).
- have a history of cardiovascular disorders.
- have diabetes (you may need to adjust your antidiabetic treatment).
- have glaucoma (increased eye pressure).
- if you are going to undergo surgery, it is advisable to stop administering the product several days before the operation.
- are taking medicines known as selective serotonin reuptake inhibitors (SSRIs).
Children and adolescents
The use of Deanxit is not recommended in children and adolescents.
Elderly
In elderly patients with dementia, a slight increase in the number of deaths has been observed in patients taking antipsychotics compared to patients not taking this medication.
Suicidal thoughts and worsening of your depression or anxiety disorder
If you are depressed and/or suffer from an anxiety disorder, you may sometimes have thoughts of harming or killing yourself. These may increase when you first start taking antidepressants, as all these medicines take time to start working, usually around two weeks, although in some cases it may be longer.
You would be more likely to have these thoughts:
- If you have previously had thoughts of killing yourself or harming yourself.
- If you are a young adult. Information from clinical trials has shown an increased risk of suicidal behavior in adults under 25 years of age with psychiatric disorders who were treated with an antidepressant.
If at any time you have thoughts of harming or killing yourself, contact your doctor or go directly to a hospital.
It may be helpful for you to tell a relative or close friendthat you are depressed or have an anxiety disorder and ask them to read this leaflet.You can ask them if they think your depression or anxiety disorder has worsened, or if they are concerned about changes in your attitude.
Manic episodes
Some patients with bipolar disorder may enter a manic phase. This is characterized by a profuse and rapid change of ideas, exaggerated joy, and excessive physical activity. In that case, it is essential that you consult your doctor; it is likely that they will change your medication.
Using Deanxit with other medicines
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Tell your doctor if you are taking any of the following medicines:
- Adrenaline, ephedrine, isoprenaline, noradrenaline, phenylephrine, and phenylpropanolamine (including some cold medicines).
- Guanethidine and similar medicines (used to lower blood pressure)
- Tricyclic antidepressants.
- Medicines that change heart rhythm (quinidine, amiodarone, sotalol, dofetilide, thioridazine, erythromycin, terfenadine, astemizole, gatifloxacin, moxifloxacin, cisapride, lithium)
- Medicines that cause an alteration of the hydro-saline balance (too little potassium or magnesium in your blood).
- Medicines that increase the concentration of Deanxit in the blood.
- Medicines that produce drowsiness (alcohol, barbiturates, and other medicines with a sedative effect).
- Lithium (for the prevention and treatment of bipolar disorder).
- Levodopa (for the treatment of Parkinson's disease).
Using Deanxit with food, drinks, and alcohol
Deanxit may increase the sedative effects of alcohol, so you may feel more drowsy. It is not recommended to consume alcohol during treatment with Deanxit.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, or think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Pregnancy
If you are pregnant or think you may be pregnant, consult your doctor. Deanxit should not be used during pregnancy unless clearly necessary.
The following symptoms may appear in newborns of mothers who have taken Deanxit during the third trimester (last three months of pregnancy): tremors, muscle stiffness and/or weakness, sleepiness, agitation, breathing problems, and difficulty feeding.
If your baby has any of these symptoms, you should contact your doctor.
Breastfeeding
If you are breastfeeding, consult your doctor. You should not use Deanxit during breastfeeding, as small amounts of the medicine may pass into breast milk.
Fertility
Animal studies have shown that Deanxit affects fertility. Please consult your doctor.
Driving and using machines
Generally, Deanxit does not cause drowsiness; however, if you feel dizzy or sleepy when you start taking this medicine, do not drive or operate tools or machines until the effects disappear.
Deanxit contains lactose
If your doctor has told you that you have an intolerance to some sugars, consult them before taking this medicine.
Deanxit contains sodium
This medicine contains less than 23 mg of sodium (1 mmol) per tablet; this is essentially "sodium-free".
3. How to take Deanxit
Follow exactly the administration instructions of this medicine given by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
The recommended dose is:
Adults
Normally two tablets a day: 1 in the morning and 1 at noon.
Your doctor may increase the dose to 2 tablets in the morning and 1 at noon.
The maximum dose is 4 tablets a day.
Maintenance dose: usually, 1 tablet in the morning.
Elderly patients (over 65 years old)
1 tablet in the morning.
Your doctor may increase the dose to 1 tablet in the morning and 1 tablet at noon.
Maintenance dose: usually, 1 tablet in the morning.
Deanxit can be taken with or without food.
Swallow the tablets with a glass of water. Do not chew them.
Duration of treatment
Generally, patients respond to Deanxit treatment quite quickly, although if you have taken the maximum dose for about a week and still do not feel better, your doctor may decide to stop the treatment.
Your doctor will indicate the duration of your treatment. Continue the treatment according to your doctor's recommendations.
Do not change the dose of the medicine without consulting your doctor.
If you take more Deanxit than you should
If you think you or anyone else may have taken more Deanxit tablets than you should, consult your doctor immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount taken. Do this even if there are no signs of discomfort or poisoning. Take the Deanxit package with you if you go to a doctor or hospital.
The symptoms of overdose include drowsiness, irritability, excitement, hallucinations, dilated pupils, rapid heart rate, difficulty urinating, dry mouth, constipation, convulsions, fever, unconsciousness, coma, difficulty breathing (blue discoloration of the skin), irregular heartbeats, heart problems that can cause difficulty breathing or swelling of the ankles, low blood pressure, metabolic acidosis, hypokalemia (low potassium levels that can cause muscle weakness, spasms, or irregular heartbeat).
If you forget to take Deanxit
If you forget to take a dose, take the next dose when it is due. Do not take a double dose to make up for the forgotten dose.
If you stop taking Deanxit
Do not stop taking Deanxit even if you start to feel better, unless your doctor has told you to do so.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you experience any of the following symptoms, you should contact your doctor or go to the hospital immediately.
Very rare (may affect up to 1 in 10,000 people):
- Unusual movements of the mouth and tongue; this may be an early sign of a disease known as tardive dyskinesia.
- High fever, unusual muscle stiffness, and disorders of consciousness, especially if it occurs with sweating and rapid heartbeat; these symptoms may be signs of a rare disease called neuroleptic malignant syndrome, which has been reported with the use of different antipsychotics.
- Yellowing of the skin and the whites of the eyes, this may mean that your liver is affected and a sign of a disease known as jaundice.
- Signs of infection such as sudden unexplained fever, sore throat, and ulcers in the mouth; these may be signs of a strongly reduced number of white blood cells and a disease known as agranulocytosis.
Frequency not known (frequency cannot be estimated from the available data):
- Suicidal thoughts or suicidal behavior, see section "Warnings and precautions".
- Blood clots in the veins, particularly in the legs (symptoms include swelling, pain, and redness in the legs), can move through the blood vessels to the lungs, causing chest pain and difficulty breathing.
The following side effects are more pronounced at the start of treatment and most of them usually disappear during continued treatment:
Frequent (may affect up to 1 in 10 people):
- Insomnia, agitation
- Drowsiness, tremors, dizziness
- Difficulty focusing on objects close to the eye (accommodation disorder)
- Dry mouth
- Constipation
- Fatigue
- Abnormal heart tracing on an electrocardiogram (ECG)
Uncommon (may affect up to 1 in 100 people)
- Nightmares, anxiety, confusion
- Fast heart rate (tachycardia), irregular heartbeat (arrhythmia)
- Abnormal liver function tests
- Rash, hair loss (alopecia)
- Muscle pain (myalgia)
- Feeling of weakness
Rare (may affect up to 1 in 1,000 people):
- Nausea
- Digestive problems or discomfort centered in the upper abdomen (dyspepsia)
Very rare (may affect up to 1 in 10,000 people):
- Low platelet count (thrombocytopenia), reduced white blood cell count (leukopenia)
- Sudden movements (dyskinesia), tremors, stiffness, and dragging (parkinsonism)
- Liver disease
Not known (frequency cannot be estimated from the available data)
- Drug withdrawal syndrome in newborns, see also section "Pregnancy, breastfeeding, and fertility"
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Medicines Monitoring System: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Deanxit
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label. The expiry date is the last day of the month shown.
This medicine does not require any special storage temperature.
Keep in the original package to protect from light.
Medicines should not be disposed of via wastewater or household waste. Return the packages and medicines you no longer need to the pharmacy's SIGRE point. Ask your pharmacist how to dispose of medicines no longer needed. This will help protect the environment.
6. Contents of the pack and other information
Composition of Deanxit
The active substances are flupentixol and melitracen.
Each Deanxit film-coated tablet contains flupentixol dihydrochloride, equivalent to 0.5 mg of flupentixol, and melitracen hydrochloride, equivalent to 10 mg of melitracen.
The other ingredients are: betadex, lactose monohydrate, cornstarch, hydroxypropylcellulose, microcrystalline cellulose, sodium croscarmellose, talc, hydrogenated vegetable oil, magnesium stearate.
Coating: poly (vinyl alcohol), macrogol 3350, talc, macrogol 6000
Colorants: titanium dioxide E171, erythrosine E127, indigo carmine E132.
Appearance of the product and contents of the pack
Deanxit film-coated tablets are pink, round, and biconvex.
Deanxit tablets are available in blister packs or plastic containers in the following pack sizes:
30, 50, and 100 in blister packs
100 in plastic containers
Not all pack sizes may be marketed
Marketing authorization holder and manufacturer
Holder:
Lundbeck España, S.A.
Av. Diagonal, 605.
E-08028 Barcelona.
Spain
Manufacturer:
- Lundbeck A/S
Ottiliavej 9
2500 Valby
Denmark
Date of last revision of this leaflet: March 2021
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to DEANXIT FILM-COATED TABLETSDosage form: CAPSULE, 5/12.5 mg/mgActive substance: amitriptyline and psycholepticsManufacturer: Kern Pharma S.L.Prescription requiredDosage form: CAPSULE, 25/10 mgActive substance: amitriptyline and psycholepticsManufacturer: Kern Pharma S.L.Prescription requiredDosage form: TABLET, 15 mgActive substance: mirtazapineManufacturer: Laboratorios Alter S.A.Prescription required
Online doctors for DEANXIT FILM-COATED TABLETS
Discuss questions about DEANXIT FILM-COATED TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











