
CIPROXINA SIMPLE 3 mg/ml OTIC SOLUTION DROPS

How to use CIPROXINA SIMPLE 3 mg/ml OTIC SOLUTION DROPS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Prospect: Information for the User
CIPROXINA Simple 3 mg/ml Ear Drops in Solution
Ciprofloxacin
Read the entire prospectus carefully before starting to use this medication, as it contains important information for you.
- Keep this prospectus, as you may need to read it again.
- If you have any doubts, consult your doctor or pharmacist.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience adverse effects, consult your doctor or pharmacist, even if they are not listed in this prospectus.
Contents of the Prospectus
- What CIPROXINA Simple is and what it is used for
- What you need to know before starting to use CIPROXINA Simple
- How to use CIPROXINA Simple
- Possible adverse effects
- Storage of CIPROXINA Simple
- Package contents and additional information
1. What CIPROXINA Simple is and what it is used for
CIPROXINA Simple contains an antibiotic (ciprofloxacin) that belongs to a group of medications called quinolones.
Antibiotics are used to treat bacterial infections and are not effective against viral infections.
It is essential to follow the instructions regarding dosage, administration interval, and treatment duration indicated by your doctor.
Do not store or reuse this medication. If you have any leftover antibiotic after completing the treatment, return it to the pharmacy for proper disposal. Do not throw medications down the drain or in the trash.
This medication is used to treat acute external otitis.
2. What you need to know before starting to use CIPROXINA Simple
Before prescribing this medication, your doctor will have examined your eardrums to ensure they are not perforated.
Do not use CIPROXINA Simple
If you are allergic to ciprofloxacin, other quinolone medications, or any of the other components of this medication (listed in section 6).
If you have a perforated or damaged eardrum.
Warnings and precautions
Consult your doctor or pharmacist before starting to use CIPROXINA Simple.
- Use this medication only in your ear(s).
- If you experience the first signs of a skin rash or any other hypersensitivity reaction, including hives, itching, or respiratory problems, discontinue treatment and consult your doctor immediately. If you have a severe allergic reaction, you may need emergency treatment.
- If your symptoms worsen or return, consult your doctor. With the use of this medication, you may become more susceptible to other infections, especially after using it for an extended period.
- If you are elderly or using medications called corticosteroids, which are used to treat conditions such as pain, inflammation, asthma, or skin problems, you have a higher risk of tendon problems during treatment with this medication. If you experience any inflammation or inflammatory condition, discontinue treatment and consult your doctor immediately.
- If you experience pain, swelling, or inflammation of the tendons during or shortly after using these drops, discontinue treatment and consult your doctor.
- A thorough medical check-up is necessary.
- If you notice excessive sensitivity to sunlight in the form of sunburn, you should also discontinue treatment.
- CIPROXINA Simple can also be used in children under 1 year of age for the treatment of acute external otitis, when your doctor considers it necessary.
- If you are in one of these situations, you may have a higher risk of tendon problems:
- Advanced age.
- Steroid treatment.
Using CIPROXINA Simple with other medications
Tell your doctor or pharmacist if you are using, have recently used, or may need to use any other medication.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
This medication is not recommended during pregnancy. Do not use CIPROXINA Simple during pregnancy or breastfeeding, unless your doctor considers it necessary.
If you are breastfeeding, do not use this medication, as it may pass into breast milk.
Driving and using machines
Treatment with this medication does not affect your ability to drive or use machines.
CIPROXINA Simple contains benzalkonium chloride
This medication contains 0.06 mg of benzalkonium chloride per ml.
Benzalkonium chloride may cause skin irritation. Do not apply to mucous membranes.
3. How to use CIPROXINA Simple
This medication should onlybe administered via the otic route, i.e., as ear drops. Follow the administration instructions for this medication exactly as indicated by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again.
Follow these instructions unless your doctor has given you different ones (such as using an otowick, a sponge placed in the ear in some cases of external otitis to facilitate absorption of the drops).
Remember to apply your medication when it is due.
Your doctor will indicate the duration of treatment with these drops. Do not discontinue treatment before, as it may affect the healing process of the affected ear.
The recommended dose is:
 Adults: 4 drops in the ear, twice a day, morning and night.
Adults: 4 drops in the ear, twice a day, morning and night.
Children:3 drops in the ear, twice a day, morning and night.
Usage recommendations:
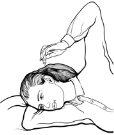
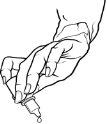
Figure 1Figure 2
- Wash your hands.
- Hold the bottle in your hand for a few minutes to warm the contents and avoid the drop being too cold when it enters your ear.
- Remove the cap.
- Hold the bottle upside down between your fingers.
- Lie on your side with the affected ear facing up (Figure 1).
- Bring the tip of the bottle close to the ear canal.
- Do not touch the ear, ear canal, or surrounding areas with the dropperbecause the drops may become infected.
- Gently press the base of the bottle to release one drop of medication at a time.
- Do not squeeze the bottle:it is designed to release a drop with gentle pressure on the base (Figure 2). Adults should apply 4 drops, and children should apply 3 drops.
- Keep your head tilted to that side for about 5 minutes to facilitate the entry of the drops into the external ear canal.
- If you are applying drops in both ears, repeat the above steps for the other ear.
- Close the bottle tightly immediately after use.
If a drop falls outside the ear, try again.
If you are using other ear drops, wait at least 5 minutes between applying CIPROXINA Simple and the other drops.
If you use more CIPROXINA Simple than you should,do not apply more drops until the next scheduled dose.
In case of overdose or accidental ingestion, consult your doctor, pharmacist, or call the Toxicology Information Service at 91 562 04 20, indicating the medication and the amount ingested.
If you forget to use CIPROXINA Simple,apply a single dose as soon as you remember, and continue with the next scheduled dose. However, if it is almost time for the next dose, do not apply the missed dose, and continue with your regular schedule. Do not apply a double dose to make up for missed doses.
If you have any further questions about using this medication, ask your doctor or pharmacist.
4. Possible adverse effects
Like all medications, CIPROXINA Simple can cause adverse effects, although not everyone will experience them.
Uncommon adverse effects (may affect up to 1 in 100 people):
- Ear effects: pain, congestion, discharge, itching.
- General effects: headache, skin inflammation, fever.
Adverse effects of unknown frequency (cannot be estimated from available data):
- Ear effects: tinnitus.
Reporting adverse effects
If you experience any adverse effects, consult your doctor or pharmacist, even if they are not listed in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medications: https://www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medication.
5. Storage of CIPROXINA Simple
Keep this medication out of sight and reach of children.
Do not use this medication after the expiration date shown on the bottle and carton after "EXP". The expiration date is the last day of the month indicated.
This medication does not require special storage conditions.
Do not refrigerate or freeze.
Keep the bottle tightly closed.
To avoid infections, discard the bottle 4 weeks after opening it for the first time.
Write the opening date of the bottle in the space provided on the carton.
Medications should not be thrown down the drain or in the trash. Deposit the packaging and any unused medications in the SIGRE collection point at your pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medications. This will help protect the environment.
6. Package contents and additional information
Composition of CIPROXINA Simple
- The active ingredient is ciprofloxacin. Each ml of solution contains 3 mg of ciprofloxacin (as hydrochloride).
- The other ingredients are benzalkonium chloride, sodium acetate trihydrate, glacial acetic acid, mannitol (E-421), disodium edetate, hydrochloric acid, and/or sodium hydroxide (to adjust the pH) and purified water.
Appearance of the product and package contents
CIPROXINA Simple is an ear drop solution presented in 5 ml plastic bottles with a screw cap.
Marketing authorization holder
Novartis Farmacéutica, S.A.
Gran Via de les Corts Catalanes, 764
08013 – Barcelona, Spain
Manufacturer
Siegfried El Masnou, S.A.
C/Camil Fabra, 58
08320 El Masnou – Barcelona, Spain
or
Novartis Manufacturing NV
Rijksweg, 14
2870 Puurs-Sint-Amands, Belgium
or
Novartis Farmacéutica, S.A.
Gran Via de les Corts Catalanes, 764
08013 Barcelona, Spain
or
Novartis Pharma GmbH
Roonstrasse 25
90429 Nuremberg, Germany
Date of the last revision of this prospectus: April 2013
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price3.76 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CIPROXINA SIMPLE 3 mg/ml OTIC SOLUTION DROPSDosage form: OTIC SOLUTION, 3 mg/mlActive substance: ciprofloxacinManufacturer: Zambon S.A.U.Prescription requiredDosage form: OTIC SOLUTION, 1.2 mg of ciprofloxacin in 0.4 mlActive substance: ciprofloxacinManufacturer: Laboratorios Salvat S.A.Prescription requiredDosage form: OTIC SOLUTION, 3 mgActive substance: ciprofloxacinManufacturer: Laboratorios Salvat S.A.Prescription required
Online doctors for CIPROXINA SIMPLE 3 mg/ml OTIC SOLUTION DROPS
Discuss questions about CIPROXINA SIMPLE 3 mg/ml OTIC SOLUTION DROPS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions








