
BRIMICA GENUAIR 340/12 micrograms powder for inhalation

How to use BRIMICA GENUAIR 340/12 micrograms powder for inhalation
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Brimica Genuair 340micrograms/12micrograms inhalation powder
aclidinium/formoterol fumarate dihydrate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Brimica Genuair and what is it used for
- What you need to know before you use Brimica Genuair
- How to use Brimica Genuair
- Possible side effects
- Storage of Brimica Genuair
- Contents of the pack and other information
Instructions for use
1. What is Brimica Genuair and what is it used for
What is Brimica Genuair
This medicine contains two active substances called aclidinium and formoterol fumarate dihydrate. Both belong to a group of medicines called bronchodilators. Bronchodilators work by relaxing the muscle in your airways, which helps to open up the airways and makes it easier for you to breathe. The Genuair inhaler delivers the active substances directly into your lungs when you inhale.
What is Brimica Genuair used for
Brimica Genuair is used in adult patients who have difficulty breathing due to a lung disease called chronic obstructive pulmonary disease (COPD), in which the airways and air sacs in the lungs are damaged or blocked. By opening up the airways, the medicine helps to relieve symptoms such as difficulty breathing. Regular use of Brimica Genuair will reduce the effects of COPD on your daily life.
2. What you need to know before you use Brimica Genuair
Do not use Brimica Genuair:
- if you are allergic to aclidinium, formoterol fumarate dihydrate, or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start using Brimica Genuair if you have any of the following symptoms/diseases:
- If you have asthma. This medicine should not be used to treat asthma.
- If you have heart problems.
- If you have epilepsy.
- If you have thyroid disorders (thyrotoxicosis).
- If you have a tumor in an adrenal gland (pheochromocytoma).
- If you have difficulty urinating or have problems due to an enlarged prostate.
- If you have an eye condition called narrow-angle glaucoma, which causes high pressure in the eye.
Stop using Brimica Genuair and seek immediate medical help if you experience any of the following symptoms
- If you notice sudden tightness in the chest, have a cough, wheezing, or difficulty breathing immediately after using the medicine. See section 4.
Brimica Genuair is used as a maintenance treatment (long-term) for COPD. This medicine should not be used to treat a sudden attack of difficulty breathing or wheezing.
If your usual symptoms of COPD (difficulty breathing, wheezing, or coughing) do not improve or worsen during treatment with Brimica Genuair, you should continue using the medicine but you should also see your doctor as soon as possible so that he can determine if you need another medication.
If you see halos around lights or images in color, have pain or discomfort in the eyes, or experience temporary blurred vision, see your doctor as soon as possible.
Dry mouth has been observed with medicines like Brimica Genuair. In the long term, dry mouth can be associated with tooth decay, so it is important that you take care of your oral hygiene.
Children and adolescents
Brimica Genuair should not be used in children or adolescents under 18 years of age.
Using Brimica Genuair with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines. If you use Brimica Genuair with other medicines, the effect of Brimica Genuair or the other medicines may be altered.
Tell your doctor or pharmacist if you are taking:
- Any medicine that may be similar to Brimica Genuair for the treatment of difficulty breathing.
- Medicines that reduce the level of potassium in the blood. These include:
- oral corticosteroids (such as prednisolone),
- diuretics (such as furosemide or hydrochlorothiazide),
- certain medicines used to treat respiratory diseases (such as theophylline).
- Medicines called beta-blockers that may be used to treat high blood pressure and other heart conditions (such as atenolol or propranolol) or to treat glaucoma (such as timolol).
- Medicines that may cause a type of change in the electrical activity of the heart called "prolongation of the QT interval" (which is seen on an electrocardiogram). These include medicines for the treatment of:
- depression (such as monoamine oxidase inhibitors or tricyclic antidepressants),
- bacterial infections (such as erythromycin, clarithromycin, or telithromycin),
- allergic reactions (antihistamines).
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor, pharmacist, or nurse for advice before using this medicine. You should not use Brimica Genuair if you are pregnant or breastfeeding unless your doctor has recommended it.
Driving and using machines
Brimica Genuair is unlikely to affect your ability to drive or use machines. However, this medicine may cause blurred vision or dizziness in some patients. If you experience any of these side effects, do not drive or use machines until the dizziness has passed and your vision has returned to normal.
Brimica Genuair contains lactose
This medicine contains lactose. If your doctor has told you that you have an intolerance to some sugars, contact them before taking this medicine.
3. How to use Brimica Genuair
Follow exactly the instructions for administration of this medicine given by your doctor or pharmacist. If you are in doubt, consult your doctor or pharmacist again.
- The recommended dose is one inhalation in the morning and one inhalation in the evening.
- You can use Brimica Genuair at any time, before or after you have eaten or drunk.
- The effects of Brimica Genuair last for 12 hours, so you should try to use Brimica Genuair at the same time every morning and evening, as this will ensure that there is always enough medicine in your body to help you breathe more easily throughout the day and night. Also, using it at the same time every day will help you remember to use it.
- The recommended dose can be used in elderly patients and in patients with kidney or liver problems. No dose adjustment is needed in these patients.
- Brimica Genuair is for inhalation use only.
- Instructions for use:see the Instructions for Use at the end of this leaflet to know how to use the Genuair inhaler. If you are in doubt about how to use Brimica Genuair, consult your doctor or pharmacist.
COPD is a long-term disease, and therefore, Brimica Genuair is for long-term use. The medicine should be used every day, twice a day, and not just when you have problems breathing or other symptoms of COPD.
If you use more Brimica Genuair than you should
If you think you have used more Brimica Genuair than you should, you are more likely to experience some of its side effects, such as blurred vision, dry mouth, nausea, tremors, headache, palpitations, or increased blood pressure; in this case, contact your doctor immediately or go to the nearest emergency department. Take the Brimica Genuair pack with you. You may need medical attention.
If you forget to use Brimica Genuair
If you forget a dose of Brimica Genuair, it should be taken as soon as possible, and the next dose should be taken at the usual time. Do not take a double dose to make up for forgotten doses.
If you stop using Brimica Genuair
This medicine is for long-term treatment. If you want to stop treatment, consult your doctor first, as your symptoms may worsen.
If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Stop using the medicine and contact your doctor immediately if you:
- experience swelling of the face, throat, lips, or tongue (with or without difficulty breathing or swallowing), hives, and severe itching of the skin (urticaria), as these may be symptoms of an allergic reaction. The frequency of this reaction cannot be estimated from the available data.
- experience tightness in the chest, cough, wheezing, or difficulty breathing immediately after using the medicine. These may be signs of a condition called "paradoxical bronchospasm", which is a severe and prolonged contraction of the muscle in the airways immediately after treatment with a bronchodilator. This reaction may occur rarely (affects 1 in 1,000 people).
Some side effects can be serious:if you experience any of these side effects, tell your doctor immediately.
Uncommon:may affect up to 1 in 100 people
- Weakness or muscle spasms and/or abnormal heart rhythm, as these may be signs of a decrease in the level of potassium in the blood
- Fatigue, increased thirst, and/or need to urinate more frequently than usual, as these may be signs of an increase in the level of sugar in the blood
- Palpitations, as this may be a sign of an unusually fast or irregular heartbeat
Rare(may affect up to 1 in 1,000 people)
- Sudden difficulty breathing or swallowing, swelling of the tongue, throat, lips, or face, rash, and/or skin itching; these may be signs of an allergic reaction
Other side effects that may occur when using Brimica Genuair:
Common:may affect up to 1 in 10 patients
- Combination of sore throat and increased mucus; these may be signs of nasopharyngitis
- Headache
- Pain when urinating and/or frequent urination; these may be signs of a urinary tract infection
- Cough
- Diarrhea
- Stuffy or runny nose, increased mucus, and/or pain or pressure in the cheeks or forehead; these may be symptoms of sinusitis
- Dizziness
- Muscle cramps
- Nausea (feeling sick)
- Difficulty sleeping
- Dry mouth
- Muscle pain
- Abscess (infection) of the gum
- High levels in the blood of a protein found in muscle tissue called creatine phosphokinase
- Tremors
- Anxiety
Uncommon
- Fast heartbeat (tachycardia)
- Abnormal or irregular heartbeat (arrhythmias)
- Chest pain or discomfort (angina pectoris)
- Blurred vision
- Changes in the tone of the voice (dysphonia)
- Difficulty urinating or feeling that the bladder is not fully empty (urinary retention)
- Abnormal electrocardiogram (prolongation of the QT interval) that may lead to an abnormal heartbeat
- Altered sense of taste (dysgeusia)
- Sore throat
- Inflammation of the mouth (stomatitis)
- Increased blood pressure
- Nervousness
- Rash
- Itching of the skin
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Brimica Genuair
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label of the inhaler, the carton, and the inhaler bag after "EXP". The expiry date is the last day of the month stated.
This medicine does not require any special storage conditions.
Keep the Genuair inhaler protected inside the closed bag until you start treatment.
Use within 60 days of opening the bag.
Do not use Brimica Genuair if you notice that the packaging is damaged or shows signs of tampering.
Once you have used the last dose, the inhaler should be discarded. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and additional information
Composition of Brimica Genuair
The active substances are aclidinium and formoterol fumarate dihydrate. Each delivered dose (the dose that comes out of the mouthpiece) contains 396 micrograms of aclidinium bromide equivalent to 340 micrograms of aclidinium and 11.8 micrograms of formoterol fumarate dihydrate.
The other component is lactose monohydrate (see the end of section 2 under the heading “Brimica Genuair contains lactose” for more information).
Appearance of the product and pack contents
Brimica Genuair is a white or almost white inhalation powder.
The Genuair inhaler is a white device with an integrated dose indicator and an orange dosing button. The mouthpiece is covered by a removable orange protective cap. It is provided in a closed aluminum foil bag containing a desiccant sachet. After removing the inhaler from the bag, the bag and the desiccant should be discarded.
Available pack sizes:
Pack containing 1 inhaler with 30 doses.
Pack containing 1 inhaler with 60 doses.
Pack containing 3 inhalers with 60 doses each.
Not all pack sizes may be marketed.
Marketing authorisation holder
Covis Pharma Europe B.V.
Gustav Mahlerplein 2
1082MA Amsterdam
Netherlands
Manufacturer
Industrias Farmacéuticas Almirall, S.A.
Ctra. de Martorell 41-61
08740 Sant Andreu de la Barca, Barcelona
Spain
For further information about this medicinal product, please contact the local representative of the marketing authorisation holder:
Belgium/Belgique/Belgien Covis Pharma Europe B.V. Tel: 80013067 | Lithuania UAB “BERLIN-CHEMIE MENARINI BALTIC” Tel: +370 52 691 947 |
| Luxembourg/Luxemburg Covis Pharma Europe B.V. Tel: 80024119 |
Czech Republic Berlin-Chemie/A.Menarini Ceska republika s.r.o. Tel: +420 267 199 333 | Hungary Berlin-Chemie/A. Menarini Kft. Tel.: +36 1799 7320 |
Denmark Covis Pharma Europe B.V. Tlf: 80711260 | Malta Covis Pharma Europe B.V. Tel: 80065149 |
Germany Berlin-Chemie AG Tel: +49 (0) 30 67070 Covis Pharma Europe B.V. Tel: +49 (0) 3031196978 | Netherlands Covis Pharma Europe B.V. Tel: 08000270008 |
Estonia OÜ Berlin-Chemie Menarini Eesti Tel: +372 667 5001 | Norway Covis Pharma Europe B.V. Tlf: 80031492 |
Greece MENARINI HELLAS AE Tel: +30 210 8316111-13 | Austria
Tel: +43 1 879 95 85-0 |
Spain Laboratorios Menarini S.A. Tel: +34-93 462 88 00 | Poland Covis Pharma Europe B.V. Tel.: 0800919353 |
France MENARINI France Tel: +33 (0)1 45 60 77 20 | Portugal
Tel: +351 210 935 500 |
Croatia Berlin-Chemie Menarini Hrvatska d.o.o. Tel: + 385 1 4821 361 | Romania Berlin-Chemie A.Menarini S.R.L. Tel: +40 21 232 34 32 |
Ireland Menarini Pharmaceuticals Ireland Ltd Tel: +353 1 284 6744 | Slovenia Berlin-Chemie / A. Menarini Distribution Tel: +386 01 300 2160 |
Iceland Covis Pharma Europe B.V. Sími: 8007279 | Slovakia Berlin-Chemie / A Menarini Distribution Tel: +421 2 544 30 730 |
Italy Laboratori Guidotti S.p.A. Tel: +39- 050 971011 | Finland Covis Pharma Europe B.V. Puh/Tel: 0800413687 |
Cyprus Covis Pharma Europe B.V. Tel: 80091079 | Sweden Covis Pharma Europe B.V. Tel: 0200898678 |
Latvia SIA Berlin-Chemie/Menarini Baltic Tel: +371 67103210 | United Kingdom (Northern Ireland) Covis Pharma Europe B.V. Tel: 08004334029 |
Date of last revision of this leaflet:
Detailed information on this medicinal product is available on the website of the European Medicines Agency: http://www.ema.europa.eu/.
Instructions for use
This section contains information on how to use the Genuair inhaler. It is important that you read this information, as Genuair may work differently from inhalers you have used before. We also provide a demonstration video of the use of the Genuair inhaler, which is available at www.genuair.com and using the code below. If you have any questions about how to use the inhaler, consult your doctor, pharmacist, or nurse.
The instructions for use are divided into the following sections:
- How to start
- Step 1: Prepare your dose
- Step 2: Inhale your medicine
- Additional information
How to start
Read these instructions for use before starting to use the medicine
Familiarize yourself with the parts of your Genuair inhaler
Disposal of unused medicine and all materials that have come into contact with it will be carried out in accordance with local regulations.
Instructions for use
How to start:
Read these instructions for use before starting to use the medicine
Familiarize yourself with the parts of your Genuair inhaler
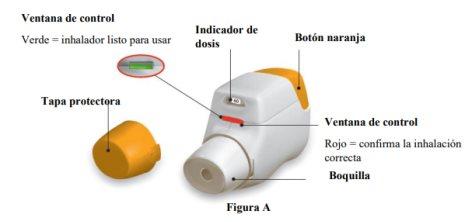
Before use:
- Before the first use, open the closed bag and remove the inhaler. Dispose of the bag and the desiccant.
- Do not press the orange button until you are ready to inhale a dose.
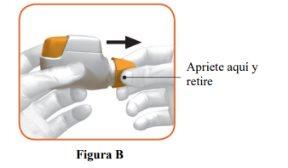
- Remove the cap by gently pressing the arrows on either side (Figure B).
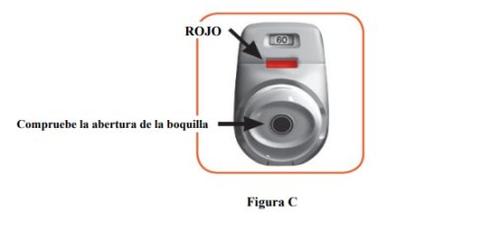
STEP 1: Prepare your dose
- Check the mouthpiece opening and make sure nothing is blocking it (Figure C).
- Check the control window (it should be red, Figure C).
- Hold the inhaler horizontally with the mouthpiece facing you and the orange button on top (Figure D).
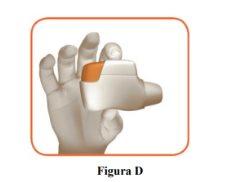
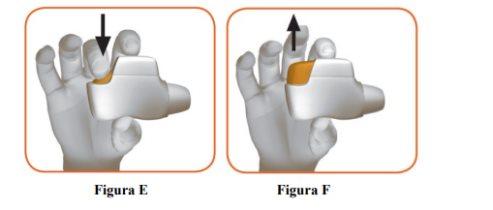
- Press the orange button down and all the way to load your dose (Figure E).
When you press the orange button down and all the way, the control window will change from red to green.
Make sure the orange button is up. Do not tilt it. |
- Release the orange button (Figure F).
Make sure to release the button so that the inhaler can work properly. |
Stop and check:
- Make sure the control window is now green (Figure G).
Your medicine is ready to be inhaled.
Go to “STEP 2: Inhale your medicine”.
Figure G
What to do if the control window remains red after pressing the button (Figure H).
The dose is not prepared. Go back to “STEP 1 Prepare your dose” and repeat steps 1.1 to 1.6. |
STEP 2: Inhale your medicine
Read the complete steps from 2.1 to 2.7 before using. Do not tilt it. |
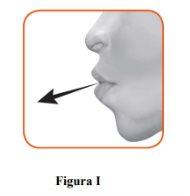
2.1 Keep the inhaler away from your mouth and exhale completely.Never exhale into the inhaler (Figure I).
2.2 Keep your head upright, place the mouthpiece between your lips, and close them tightly around the mouthpiece (Figure J).
Do not keep the orange button pressed while inhaling. |
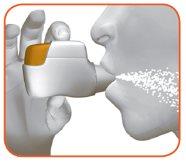
Figure J
2.3 Take a strong and deep breaththrough your mouth. Continue breathing in for as long as possible.
A “click” will let you know that you have inhaled correctly. Continue inhaling for as long as possible after hearing the “click”. Some patients may not hear the “click”. Use the control window to make sure you have inhaled correctly. |
2.4 Remove the inhaler from your mouth.
2.5 Hold your breath for as long as possible.
2.6 Breathe out slowly away from the inhaler.
Some patients may experience a gritty sensation in the mouth or a slightly sweet or bitter taste. Do not inhale an extra dose if you do not notice any taste or feel nothing after inhaling. |
Stop and check:
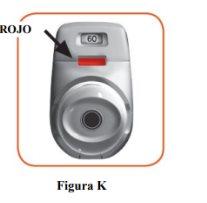
2.7 Make sure the control window is now red (Figure K). This means you have inhaled your medicine correctly.
What to do if the control window remains green after inhaling (Figure L).
Figure L This means you have not inhaled your medicine correctly. Go back to “STEP 2 Inhale your medicine” and repeat steps 2.1 to 2.7. If the control window remains green, you may have forgotten to release the orange button before inhaling, or you may not have inhaled strongly enough. If this happens, try again. Make sure you have released the orange button and that you have exhaled completely. Then take a strong and deep breath through the mouthpiece. Please contact your doctor if the control window remains green after several attempts. |
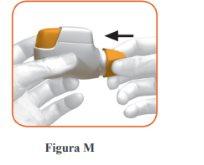
Replace the protective cap on the mouthpiece after each use (Figure M), to prevent contamination of the inhaler with dust or other materials. You must discard your inhaler if you lose the cap.
Additional information:
What should you do if you accidentally load a dose?
Keep your inhaler with the protective cap in place until it is time to inhale your medicine, then remove the cap and start at step 1.6.
How does the dose indicator work?
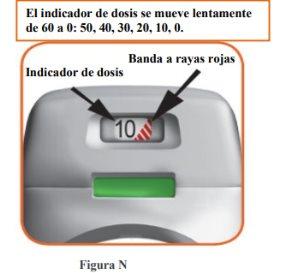
- The dose indicator shows the total number of doses left in the inhaler (Figure N).
- On the first use, each inhaler contains at least 60 or 30 doses, depending on the pack size.
- Each time a dose is loaded by pressing the orange button, the dose indicator moves slightly to the next number (50, 40, 30, 20, 10, or 0).
When should you get a new inhaler?
You should get a new inhaler:
- If your inhaler appears to be damaged or you lose the cap, or
- When a red striped band appears on the dose indicator, this means you are approaching the last dose (Figure N), or
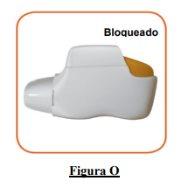
- If your inhaler is empty (Figure O).
How do you know if your inhaler is empty?
When the orange button does not return to its fully upper position and remains stuck in a middle position, it has reached the last dose (Figure O). Even when the orange button is stuck, you can still inhale the last dose. After that, the inhaler cannot be used again and you should start using a new inhaler.
How should you clean the inhaler?
NEVER use water to clean the inhaler, as this could damage your medicine.
If you want to clean your inhaler, simply wipe the outside of the mouthpiece with a dry cloth or paper towel.
- Country of registration
- Average pharmacy price70.25 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BRIMICA GENUAIR 340/12 micrograms powder for inhalationDosage form: PULMONARY INHALATION, 340/12 microgramsActive substance: formoterol and aclidinium bromideManufacturer: Covis Pharma Europe B.V.Prescription requiredDosage form: PULMONARY INHALATION, 55 MICROGRAMS/22 MICROGRAMSActive substance: vilanterol and umeclidinium bromideManufacturer: Glaxosmithkline (Ireland) LimitedPrescription requiredDosage form: PULMONARY INHALATION, 0.5 mg/2.5 mgActive substance: salbutamol and ipratropium bromideManufacturer: Genetic S.P.A.Prescription required
Online doctors for BRIMICA GENUAIR 340/12 micrograms powder for inhalation
Discuss questions about BRIMICA GENUAIR 340/12 micrograms powder for inhalation, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions


















