BERINERT 3000 IU POWDER AND SOLVENT FOR SUBCUTANEOUS INJECTION

How to use BERINERT 3000 IU POWDER AND SOLVENT FOR SUBCUTANEOUS INJECTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Berinert 3000 UI
Powder and solvent for solution for subcutaneous injection
Human C1 esterase inhibitor
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information:
- What is Berinert and what is it used for
- What you need to know before you use Berinert
- How to use Berinert
- Possible side effects
- Storage of Berinert
- Contents of the pack and other information
1. What is Berinert and what is it used for
What is Berinert?
Berinert is presented as a powder and solvent. The prepared solution should be administered by subcutaneous injection.
Berinert is prepared from human plasma (the liquid part of the blood). The active substance is human C1 esterase inhibitor protein, obtained from plasma.
What is Berinert used for?
Berinert is used for the prevention of recurrent attacks of hereditary angioedema (HAE) in adolescent and adult patients. Hereditary angioedema is a congenital vascular disease. It is not an allergic disease. HAE is caused by insufficient, absent, or defective synthesis of C1 esterase inhibitor, which is an important protein. The disease is characterized by the following symptoms:
- sudden swelling of hands and feet,
- sudden swelling of the face with a feeling of tightness,
- swelling of the eyelids, lips, possible swelling of the larynx (voice organ) with breathing difficulties,
- swelling of the tongue,
- colic-like pain in the abdominal region.
Generally, all parts of the body can be affected.
2. What you need to know before you use Berinert
The following sections contain information that your doctor should consider before administering Berinert to you.
Do not use Berinert:
- If you have experienced life-threatening immediate hypersensitivity reactions, including anaphylaxis, to C1 esterase inhibitor or to any of the other components of this medicine (listed in section 6).
Tell your doctor or pharmacist if you are allergic to any medicine or any food.
Warnings and precautions
Consult your doctor or pharmacist before starting to use Berinert,
- If you have a history of blood coagulation problems. Blood clots have occurred in patients treated with Berinert intravenously. The use of Berinert at very high doses in diseases other than HAE could increase the risk of blood clots. However, in the case of subcutaneous Berinert, there is no established relationship with blood clots at the recommended dose. Consult your doctor if you have a history of heart or blood vessel disease, infarction, blood clots, or thick blood, a permanent catheter/device in one of your veins, or have been immobilized for some time. These situations may increase the risk of developing a blood clot after using Berinert. Also, inform your doctor about the medications you are using, as some medications, such as contraceptives or certain androgens, may increase the risk of developing a blood clot.
Your doctor will carefully weigh the benefits of treatment with Berinert against the risk of these complications.
Viral safety
When administering medicines derived from human blood or plasma, certain measures must be taken to prevent infections from being passed on to patients. Such measures include:
- careful selection of blood and plasma donors to exclude those who are at risk of being carriers of infectious diseases, and
- testing for specific virus and infection markers in individual donations and in plasma pools.
Manufacturers of these products also include stages in the processing of blood or plasma to eliminate/inactivate viruses. Despite this, when administering medicines derived from human blood or plasma, the possibility of transmitting infectious agents cannot be entirely excluded. This also applies to emerging or unknown viruses or other types of infections.
The measures taken are considered effective for enveloped viruses such as human immunodeficiency virus (HIV, the AIDS virus), hepatitis B virus, hepatitis C virus (liver inflammation), and for non-enveloped viruses such as hepatitis A virus (liver inflammation) and parvovirus B19.
It is possible that your doctor may recommend vaccination against hepatitis A and B if you are treated periodically/repeatedly with human plasma-derived medicines.
It is strongly recommended that each time Berinert is administered, the administration date, batch number, and injected volume be recorded.
Using Berinert with other medicines
- Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines, including those without a prescription.
- Berinert should not be mixed with other medicines and diluents in the same syringe.
Pregnancy and breastfeeding
- If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Driving and using machines
Berinert does not affect your ability to drive or use machines.
Berinert contains sodium
This medicine contains 29 mg of sodium (main component of cooking/table salt) in each vial. This is equivalent to 1.5% of the maximum recommended daily sodium intake for an adult.
3. How to use Berinert
Berinert is indicated for self-administration by subcutaneous injection. You or your caregiver should be trained, as necessary, on how to administer Berinert.
Dosage
The recommended dose of Berinert is 60 IU/kg body weight.
Pediatric population
The recommended dose is the same as for adults.
If you use more Berinert than you should
No cases of overdose have been reported.
Reconstitution and administration
If your doctor decides that you can treat yourself at home, they will give you detailed instructions. You will be given a diary where you will record each injection administered at home, which you will bring with you every time you visit the doctor. You or your caregiver will be regularly reviewed on how to administer the injections to ensure that they are done correctly over time.
General instructions
- The powder must be dissolved and withdrawn from the vial under aseptic conditions. Use the syringe provided with the product.
- The prepared solution should be clear and transparent to slightly opalescent. After filtering or transferring the solution (see below), it should be visually checked for particles or discoloration before administration.
- Do not use the solution if it is visibly cloudy or contains particles or residues.
- Any unused medicine or residual material should be disposed of in accordance with local regulations and following your doctor's instructions.
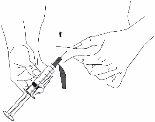
Reconstitution
Before opening any vial, allow the Berinert powder and solvent to reach room temperature. To do this, you can leave the vials at room temperature for about an hour or hold them in your closed hands for a few minutes. DO NOT expose the vials to direct heat. The vials should not be heated to a temperature above body temperature (37 °C).
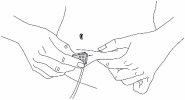
Carefully remove the protective caps from the solvent vial and the powder vial. Clean the exposed rubber stoppers of both vials with an alcohol swab and let them dry. You can now transfer the solvent to the powder vial using the included administration system (Mix2Vial). Please follow the instructions below:
|
|
|
|
|
|
|
|
|
Discard the solvent vial with the attached blue Mix2Vial adapter. |
|
|
|
|
Transfer and administration
8 |
|
9 |
|
Administration
Self-administration (subcutaneous administration)
Your doctor will teach you how to administer Berinert safely. Once you know how to self-administer the medicine, follow the instructions provided below.
Table 2. Instructions for self-administration of Berinert
Step 1: Attach the accessories Take the Berinert syringe, the following disposable accessories, and other items (needles or other containers, treatment diary or logbook):
| |
Step 2: Clean the surface
| |
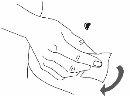
Step 3: Wash your hands
| |
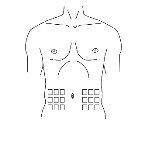
Step 4: Prepare the injection site
|
Figure 1
Figure 2 |
Step 5: Injection into the abdominal area As indicated by your healthcare professional:
Injection with a hypodermic needle:
Injection with a subcutaneous infusion set:
|
Figure 3 |
Figure 4 | |
Step 6: Clean up
| |
Step 7: Record the treatment
|
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Immediately contact your doctor
Side effects with Berinert are rare.
The following side effects have been observed very frequently (may affect more than 1 in 10 people):
- Reactions at the injection site (bruising, feeling of cold, suppuration, erythema, hematoma, hemorrhage, hardening, edema, pain, pruritus, rash, scarring, swelling, urticaria, heat).
- Nasopharyngitis (stuffy nose, sneezing, runny eyes).
The following side effects have been observed frequently (may affect up to 1 in 10 people):
- Hypersensitivity or allergic reactions (such as hypersensitivity, pruritus, rash, and urticaria)
- Dizziness
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Berinert
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date which is stated on the label and carton after EXP.
- Do not store above 30 °C.
- Do not freeze.
- Store the vial in the outer packaging to protect it from light.
- Berinert does not contain preservatives, so the prepared solution should be used immediately.
- If the prepared solution is not administered immediately, it should be used within 8 hours and only stored in the vial.
6. Container Contents and Additional Information
Berinert Composition
The active ingredient is:
Human C1 esterase inhibitor (3,000 IU/vial; after reconstitution with 5.6 ml of water for injectable preparations 500 IU/ml).
The other components are:
Glycine, sodium chloride, sodium citrate.
Solvent:water for injectable preparations.
Product Appearance and Container Contents
Berinert is presented as a white powder and is supplied with water for injectable preparations as a solvent.
The prepared solution should be transparent and clear to slightly opalescent.
Presentation
A container contains:
1 vial with powder
1 vial with 5.6 ml of water for injectable preparations
1 transfer device with 20/20 filter
Administration equipment (inner box):
1 disposable 10 ml syringe
1 hypodermic needle
1 subcutaneous injection device (butterfly)
2 alcohol swabs
1 dressing
Multiple packaging of 5 x 3,000 IU, including a box with 5 administration equipment.
Multiple packaging of 20 x 3,000 IU, including 4 boxes with 5 administration equipment.
Only some pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
CSL Behring GmbH
Emil-von-Behring-Strasse 76
35041 Marburg
Germany
For further information about this medicinal product, please contact the local representative of the marketing authorization holder:
CSL Behring S.A.
c/ Tarragona 157, 18th floor
08014 Barcelona
Spain
This medicinal product is authorized in the Member States of the European Economic Areaand in the United Kingdom (Northern Ireland)under the following names:
Berinert 3000 I.E. Powder and solvent for solution for injection Austria
Berinert 3000 IE, powder and solvent for solution for injection Belgium, Netherlands
Berinert 3000 Cyprus, Germany, Greece, Poland, Portugal
???????? 3000, ???? ? ???????????
?? ??????????? ???????
C1- ????????? ?????????, ??????? Bulgaria
Berinert 3000 IU Czech Republic, Slovakia
Berinert 3000 IU powder and solvent for solution for injection _______________________________ Croatia
Berinert Denmark, Italy
Berinert SC Estonia
Berinert 3000 IU, powder and solvent for solution for injection Finland
Berinert 3000 UI, powder and solvent for injectable solution France, Luxembourg
Berinert 3000 NE powder and solvent for injectable solution Hungary
Berinert 3000 a.e. powder and solvent, solution for injection Iceland
Human C1-esterase inhibitor CSL Behring 3000 TV powder and solvent for injectable solution Lithuania
Berinert 3000 IU powder and liquid for injectable solution, solution Norway
Berinert 3000 3000 UI, powder and solvent for injectable solution Romania
Berinert 3000 i.e. powder and vehicle for solution for injection Slovenia
Berinert 3000 UI powder and solvent for subcutaneous injectable solution Spain
Berinert 3000 IE, powder and liquid for injectable solution, solution Sweden
Berinert 3000 IU Powder and solvent for solution for injection _____________________ United Kingdom, Malta, Ireland
Date of last revision of this leaflet:October 2021
Detailed and updated information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
Thisinformation is intended only for healthcare professionals
QUALITATIVE AND QUANTITATIVE COMPOSITION
The potency of human C1 esterase inhibitor is expressed in International Units (IU), which is related to the current WHO standard for C1 esterase inhibitor products.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BERINERT 3000 IU POWDER AND SOLVENT FOR SUBCUTANEOUS INJECTIONDosage form: INJECTABLE, 1500 IUActive substance: c1-inhibitor, plasma derivedManufacturer: Csl Behring GmbhPrescription requiredDosage form: INJECTABLE, 2000 IUActive substance: c1-inhibitor, plasma derivedManufacturer: Csl Behring GmbhPrescription requiredDosage form: INJECTABLE, 500 UActive substance: c1-inhibitor, plasma derivedManufacturer: Csl Behring GmbhPrescription required
Online doctors for BERINERT 3000 IU POWDER AND SOLVENT FOR SUBCUTANEOUS INJECTION
Discuss questions about BERINERT 3000 IU POWDER AND SOLVENT FOR SUBCUTANEOUS INJECTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions




 1
1 2
2 3
3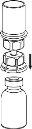 4
4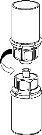 5
5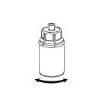 6
6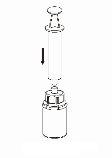 7
7