ALPROLIX 3,000 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION

How to use ALPROLIX 3,000 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
ALPROLIX 250UI powder and solvent for solution for injection
ALPROLIX 500UI powder and solvent for solution for injection
ALPROLIX 1000UI powder and solvent for solution for injection
ALPROLIX 2000UI powder and solvent for solution for injection
ALPROLIX 3000UI powder and solvent for solution for injection
eftrenonacog alfa recombinant coagulation factor IX, Fc fusion protein
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is ALPROLIX and what is it used for
- What you need to know before you use ALPROLIX
- How to use ALPROLIX
- Possible side effects
- Storage of ALPROLIX
- Contents of the pack and other information
- Instructions for preparation and administration
1. What is ALPROLIX and what is it used for
ALPROLIX contains the active substance eftrenonacog alfa, a recombinant coagulation factor IX, Fc fusion protein. Factor IX is a protein that is naturally produced by the body and is necessary for blood to clot and stop bleeding.
ALPROLIX is a medicine used to treat and prevent bleeding in patients of all ages with hemophilia B (a hereditary bleeding disorder caused by a deficiency of factor IX).
ALPROLIX is prepared using recombinant technology without the addition of any human or animal-derived components in the manufacturing process.
How ALPROLIX works
In patients with hemophilia B, factor IX is missing or does not work properly. This medicine is used to replace the missing or deficient factor IX. ALPROLIX increases factor IX concentrations in the blood and temporarily corrects the tendency to bleed. The Fc fusion protein in this medicine prolongs the duration of action of the medicine.
2. What you need to know before you use ALPROLIX
Do not use ALPROLIX
- if you are allergic to eftrenonacog alfa or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start using ALPROLIX.
- There is a small chance that you may have a severe allergic reaction (anaphylactic reaction) to ALPROLIX. Signs of allergic reactions include generalized itching, hives, feeling of tightness in the chest, difficulty breathing, and low blood pressure. If you experience any of these symptoms, stop the injection immediately and contact your doctor. Due to the risk of allergic reactions with factor IX, your first administrations of ALPROLIX should be performed under medical observation in a place where adequate medical care can be provided in case of allergic reactions.
- Talk to your doctor if you think you are not getting control of bleeding with the dose you are receiving, as there may be several reasons for this. For example, the formation of antibodies (also known as inhibitors) against factor IX is a known complication that can occur during treatment of hemophilia B. Antibodies prevent the treatment from working properly. Your doctor will check if this is the case. Do not increase the total dose of ALPROLIX to control bleeding without talking to your doctor first.
Patient with a factor IX inhibitor may have a higher risk of anaphylaxis during future treatments with factor IX. Therefore, if you experience allergic reactions such as those described above, you should be tested for the presence of an inhibitor.
Factor IX medicines may increase the risk of unwanted blood clots in your body, especially if you have risk factors for developing blood clots. Symptoms of a possible unwanted blood clot may include: pain and/or tenderness along a vein, unexpected swelling of an arm or leg, or sudden shortness of breath or difficulty breathing.
Cardiovascular disorders
If you have been told that you have a heart condition or are at risk of having one, be extra careful when using factor IX and talk to your doctor.
Catheter-related complications
If you need a central venous access device (CVAD), the risk of CVAD-related complications, including local infections, bacteria in the blood, and blood clots at the catheter insertion site, should be considered.
Documentation
We strongly recommend that every time ALPROLIX is administered, the name and batch number of the product are recorded.
Other medicines and ALPROLIX
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Driving and using machines
ALPROLIX has no influence on the ability to drive and use machines.
ALPROLIX contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per vial, which is essentially “sodium-free”. In case of treatment with several vials, the total sodium content should be taken into account.
3. How to use ALPROLIX
Treatment with ALPROLIX will be started by a doctor who is experienced in the care of patients with hemophilia. Follow exactly the administration instructions for this medicine given by your doctor (see section 7). If you are unsure, talk to your doctor, pharmacist, or nurse.
ALPROLIX is given by injection into a vein. You or another person can administer it after receiving the necessary training. Your doctor will decide the dose (in International Units or “IU”) you will receive. The dose will depend on your individual needs for factor IX replacement treatment and whether it is used for prevention or treatment of bleeding. Talk to your doctor if you think you are not getting control of bleeding with the dose you are receiving.
How often you need an injection will depend on how well the medicine is working for you. Your doctor will perform the relevant laboratory tests to ensure you have adequate factor IX concentrations in the blood.
Treatment of bleeding
The dose of ALPROLIX is calculated based on your body weight and the desired factor IX concentrations. The target factor IX concentrations depend on the severity and location of the bleeding.
Prevention of bleeding
If you are using ALPROLIX to prevent bleeding, your doctor will calculate the dose.
The usual dose of ALPROLIX is 50 IU per kilogram of body weight, administered once a week, or 100 IU per kilogram of body weight, administered once every 10 days. Your doctor may adjust the dose or interval. In some cases, especially in younger patients, shorter dosing intervals or higher doses may be necessary.
Use in children and adolescents
ALPROLIX can be used in children and adolescents of all ages. In children under 12 years of age, higher doses or more frequent injections may be necessary, and the usual dose is 50 to 60 IU per kilogram of body weight, administered once every 7 days.
If you use more ALPROLIX than you should
Tell your doctor as soon as possible. Follow exactly the administration instructions for ALPROLIX given by your doctor. If you are unsure, talk to your doctor, pharmacist, or nurse.
If you forget to use ALPROLIX
Do not take a double dose to make up for forgotten doses. Take your dose as soon as you remember and then continue with your normal dosing schedule. If you are unsure, talk to your doctor, pharmacist, or nurse.
If you stop using ALPROLIX
Do not stop using ALPROLIX without talking to your doctor. If you stop using ALPROLIX, you may no longer be protected against bleeding or an existing bleed may not stop.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If a severe allergic reaction (anaphylactic reaction) occurs, the injection should be stopped immediately. Contact your doctor immediately if you experience any of the following symptoms of allergic reactions: swelling of the face, rash, generalized itching, hives, feeling of tightness in the chest, difficulty breathing, and low blood pressure.
In children who have not received prior treatment with factor IX-containing medicines, it is common for inhibitors to form (in up to 1 in 10 patients) (see section 2). If this happens, the medicine may stop working properly, and your child may experience persistent bleeding. If this happens, contact your doctor immediately.
The following side effects may occur with this medicine.
Common side effects (may affect up to 1 in 10 people):headache, numbness or tingling in the mouth, pain in the side with blood in the urine (obstructive uropathy), and redness at the injection site.
Children who have not received prior treatment with factor IX-containing medicines: factor IX inhibitors, hypersensitivity.
Uncommon side effects (may affect up to 1 in 100 people):dizziness, altered taste, bad breath, feeling tired, pain at the injection site, rapid heartbeat, blood in the urine (hematuria), pain in the side (kidney colic), low blood pressure, and decreased appetite.
Rare side effects (frequency cannot be estimated from the available data):severe allergic reaction and potentially life-threatening allergic reaction (anaphylactic shock).
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is possible that they are not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of ALPROLIX
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and on the label of the vial after “EXP”. The expiry date refers to the last day of the month shown. Do not use this medicine if it has been stored at room temperature for more than 6 months.
Store in a refrigerator (2°C - 8°C). Do not freeze. Keep the vial in the outer carton in order to protect from light.
Alternatively, ALPROLIX can be stored at room temperature (up to 30°C) for a single period not exceeding 6 months. Record the date when ALPROLIX is removed from the refrigerator and stored at room temperature on the carton. After storage at room temperature, the medicine should not be returned to the refrigerator.
Once ALPROLIX has been prepared, it should be used immediately. If it is not possible to use the prepared solution immediately, it should be used within 6 hours if stored at room temperature. Do not refrigerate the solution after preparation. Protect the solution from direct sunlight.
The prepared solution should be clear to slightly pearlescent (opalescent) and colorless. Do not use this medicine if you notice it is cloudy or contains visible particles.
This medicine is for single use only.
Dispose of any unused solution properly. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
ALPROLIX Composition
Powder:
- The active ingredient is eftrenonacog alfa (recombinant coagulation factor IX, Fc fusion protein). Each vial of ALPROLIX contains nominally 250, 500, 1,000, 2,000, or 3,000 IU of eftrenonacog alfa.
- The other components are sucrose, L-histidine, mannitol, polysorbate 20, sodium hydroxide, and hydrochloric acid. See section 2 if you are on a low-sodium diet.
Solvent:
5 ml of sodium chloride and water for injections
Appearance and Container Contents of the Product
ALPROLIX is presented as a powder and solvent for solution for injection. The powder is a white to off-white powder or cake. The supplied solvent for preparation of the solution is a clear and colorless solution. After preparation, the solution is clear to slightly opalescent and colorless.
Each ALPROLIX container contains 1 vial of powder, 5 ml of solvent in a prefilled syringe, 1 plunger rod, 1 vial adapter, 1 infusion set, 2 alcohol swabs, 2 band-aids, and 1 gauze.
Marketing Authorization Holder and Manufacturer
Swedish Orphan Biovitrum AB (publ)
SE-112 76 Stockholm
Sweden
Phone: +46 8 697 20 00
Date of Last Revision of this Leaflet: 01/2024
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu. There are also links to other websites on rare diseases and orphan medicines.
Turn the leaflet over to consult section 7. Preparation and Administration Instructions
- Preparation and Administration Instructions
The following procedure describes the preparation and administration of ALPROLIX.
ALPROLIX is administered by intravenous (IV) injection after dissolving the injectable powder with the supplied solvent in the prefilled syringe. The ALPROLIX container contains:
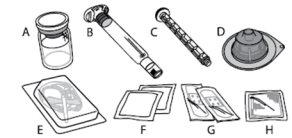

ALPROLIX should not be mixed with other injectable solutions or infusions.
Wash your hands before opening the container.
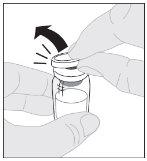
Preparation:
| |
| |
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
Do not shake. |
|
| |
Note: If using more than one vial of ALPROLIX per injection, each vial should be prepared separately according to the previous instructions (steps 1 to 13) and the solvent syringe should be removed, leaving the vial adapter in place. A single larger luer lock syringe can be used to withdraw the prepared contents of each vial. |
|
|
|
Note: If the solution is not to be used immediately, the syringe cap should be carefully replaced over the syringe tip. Do not touch the syringe tip or the inside of the cap. After preparation, ALPROLIX can be stored at room temperature for up to 6 hours before administration. After this time, the prepared ALPROLIX solution should be discarded. Protect it from direct sunlight. |
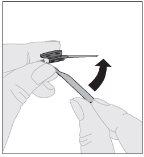
Administration (Intravenous Injection):
ALPROLIX should be administered using the provided infusion set (E).
|
|
| |
| |
| |
|
|
|
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ALPROLIX 3,000 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTIONDosage form: INJECTABLE, 1,000 IUActive substance: coagulation factor IXManufacturer: Swedish Orphan Biovitrum Ab (Publ)Prescription requiredDosage form: INJECTABLE, 2,000 IUActive substance: coagulation factor IXManufacturer: Swedish Orphan Biovitrum Ab (Publ)Prescription requiredDosage form: INJECTABLE, 250 IUActive substance: coagulation factor IXManufacturer: Swedish Orphan Biovitrum Ab (Publ)Prescription required
Online doctors for ALPROLIX 3,000 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Discuss questions about ALPROLIX 3,000 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions





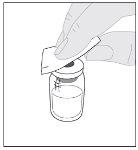
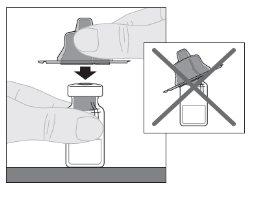
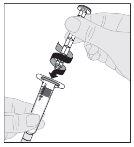
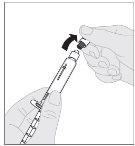
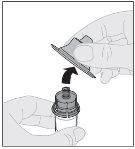
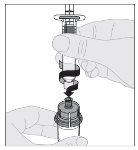
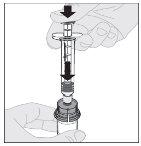
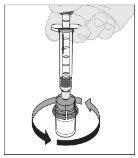
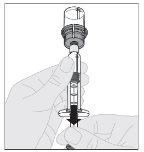
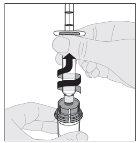
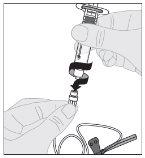
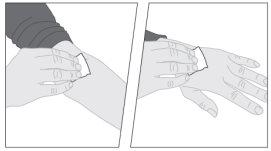 If necessary, apply a tourniquet and prepare the injection site by cleaning the skin well with the other alcohol swab provided in the container.
If necessary, apply a tourniquet and prepare the injection site by cleaning the skin well with the other alcohol swab provided in the container.