
ZEPOSIA 0,23 MG/0,46 MG CAPSULAS DURAS

Cómo usar ZEPOSIA 0,23 MG/0,46 MG CAPSULAS DURAS
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Zeposia 0,23mgcápsulas duras
Zeposia 0,46mgcápsulas duras
Zeposia 0,92mgcápsulas duras
ozanimod
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Zeposia y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Zeposia
- Cómo tomar Zeposia
- Posibles efectos adversos
- Conservación de Zeposia
- Contenido del envase e información adicional
1. Qué es Zeposia y para qué se utiliza
Zeposia contiene el principio activo ozanimod que pertenece a un grupo de medicamentos que pueden reducir el número de glóbulos blancos (linfocitos) que circulan libremente en el organismo.
Zeposia está indicado para las siguientes enfermedades:
- esclerosis múltiple
- colitis ulcerosa.
Esclerosis múltiple
Zeposia está indicado para el tratamiento de pacientes adultos con esclerosis múltiple remitente‑recurrente (EMRR) con enfermedad activa.
- La esclerosis múltiple (EM) es una enfermedad en la que el sistema inmunitario (las defensas del organismo, incluidos los glóbulos blancos) ataca erróneamente a la vaina protectora que envuelve los nervios del cerebro y la médula espinal. Esto impide que los nervios funcionen correctamente y puede producir síntomas como entumecimiento, dificultad para andar y problemas de visión y de equilibrio.
- En la esclerosis múltiple remitente‑recurrente, los ataques a las células nerviosas van seguidos de periodos de recuperación. Los síntomas pueden desaparecer durante los periodos de recuperación, aunque pueden permanecer algunos de ellos.
Zeposia ayuda a combatir los ataques sobre los nervios impidiendo que ciertos glóbulos blancos lleguen al cerebro y a la médula espinal donde podrían causar inflamación y dañar la vaina protectora de los nervios.
Colitis ulcerosa
Zeposia está indicado para el tratamiento de pacientes adultos con colitis ulcerosa (CU) activa de moderada a grave.
- La colitis ulcerosa es una enfermedad inflamatoria del intestino. Si tiene colitis ulcerosa, primero se le administrarán otros medicamentos. Si no responde lo suficientemente bien o es intolerante a estos medicamentos, se le puede administrar Zeposia para reducir los signos y síntomas de su enfermedad.
Zeposia ayuda a reducir la inflamación en la colitis ulcerosa impidiendo que ciertos glóbulos blancos lleguen al revestimiento intestinal.
2. Qué necesita saber antes de empezar a tomar Zeposia
No tome Zeposia:
- si es alérgico a ozanimod o a alguno de los demás componentes de este medicamento (incluidos en la sección 6);
- si su profesional sanitario le ha dicho que su sistema inmunitario está muy debilitado
- si ha tenido un ataque al corazón, una angina de pecho, un ictus o un mini‑ictus (un ataque isquémico transitorio [AIT]) o ciertos tipos de insuficiencia cardiaca grave en los últimos 6 meses
- si tiene ciertos tipos de latido cardiaco irregular o anormal (arritmia); su médico comprobará su corazón antes de iniciar el tratamiento
- si tiene una infección grave como hepatitis o tuberculosis
- si tiene cáncer
- si tiene problemas hepáticos graves
- si está embarazada o tiene posibilidad de quedarse embarazada y no está utilizando un método anticonceptivo eficaz.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a tomar Zeposia si:
- tiene la frecuencia cardiaca lenta o toma o ha tomado recientemente medicamentos que ralentizan la frecuencia cardiaca (como betabloqueantes o bloqueantes de los canales de calcio)
- tiene problemas respiratorios graves cuando duerme (apnea del sueño grave) sin tratar
- tiene problemas de hígado
- tiene una infección
- tiene niveles bajos de un tipo de glóbulos blancos llamados linfocitos
- nunca ha tenido, o no está seguro si ha tenido, varicela
- ha recibido recientemente o tiene previsto recibir una vacuna
- usted u otras personas observan un empeoramiento de los síntomas de la EM así como algún síntoma nuevo o poco habitual. Estos síntomas se pueden deber a una infección rara del cerebro llamada leucoencefalopatía multifocal progresiva (LMP). Si se confirma la LMP, su médico interrumpirá el tratamiento con Zeposia. Sin embargo, algunas personas pueden sufrir una reacción al retirar Zeposia. Esta reacción (conocida como SIRI o síndrome inflamatorio de reconstitución inmunitaria) puede hacer que su enfermedad empeore, incluido el empeoramiento de la función cerebral
- ha tenido alguna vez problemas de visión u otros síntomas de acumulación de líquido en la zona central de la retina llamada mácula (una enfermedad llamada edema macular)
- tiene inflamación ocular (uveítis)
- tiene diabetes (que puede producir problemas en los ojos)
- tiene enfermedad pulmonar grave (fibrosis pulmonar o enfermedad pulmonar obstructiva crónica).
Antes de empezar a tomar Zeposia, su médico le realizará un electrocardiograma (ECG) para comprobar su corazón.
Si tiene ciertas afecciones cardiacas, su médico le supervisará durante al menos las primeras 6 horas tras la primera dosis.
Dado que Zeposia puede aumentar la tensión arterial, puede que su médico quiera controlarle la tensión arterial periódicamente.
Durante el tratamiento con Zeposia, si experimenta náuseas sin causa aparente, vómitos, dolor en el lado derecho del área estomacal (dolor abdominal), cansancio, pérdida del apetito, coloración amarillenta en la piel o en los ojos (ictericia) y/u orina oscura, informe a su médico de inmediato. Estos síntomas pueden deberse a un problema hepático.
Su médico pedirá análisis de sangre para controlar la función hepática antes, durante y después del tratamiento. Si los resultados de los análisis indican un problema hepático, es posible que deba interrumpir el tratamiento con Zeposia.
Mientras toma Zeposia (y durante un periodo de hasta 3 meses después de dejar de tomarlo), puede presentar infecciones con mayor facilidad. Cualquier infección que ya tenga puede empeorar. Consulte a su médico si presenta una infección.
Durante el tratamiento con Zeposia, si presenta alteraciones de la visión, debilidad progresiva, torpeza, pérdida de memoria o confusión, o si tiene EM y cree que su enfermedad está empeorando progresivamente, hable con su médico inmediatamente. Estos síntomas pueden deberse a LMP, una infección cerebral poco común que puede provocar una discapacidad grave o la muerte.
Durante el tratamiento con Zeposia, si presenta un dolor de cabeza intenso, confusión, o convulsiones (crisis epilépticas) y pérdida de visión, consulte a su médico inmediatamente. Estos síntomas se pueden deber a un síndrome llamado síndrome de encefalopatía posterior reversible (SEPR).
Dado que Zeposia puede aumentar el riesgo de cáncer de piel, debe limitar su exposición a la luz solar y a la luz ultravioleta (UV) utilizando ropa protectora y aplicándose crema solar regularmente (con un alto factor de protección solar).
Mujeres con posibilidad de quedarse embarazadas
Si se utiliza durante el embarazo, Zeposia puede dañar al feto. Antes de iniciar el tratamiento con Zeposia, su médico le informará sobre los riesgos y le pedirá que se haga una prueba de embarazo para asegurarse de que no está embarazada. Su médico le entregará una tarjeta en la que se explica por qué no debe quedarse embarazada mientras esté tomando Zeposia. También le indica qué debe hacer para evitar quedarse embarazada mientras está tomando Zeposia. Debe utilizar un método anticonceptivo efectivo durante el tratamiento y durante 3 meses después de dejar de tomar el tratamiento (ver sección “Embarazo y lactancia”).
Si cualquiera de estos puntos es aplicable en su caso, informe a su médico o farmacéutico antes de tomar Zeposia.
Empeoramiento de la EM tras la interrupción del tratamiento con Zeposia
Informe a su médico de inmediato si cree que su EM empeora tras interrumpir el tratamiento con Zeposia (ver “Si interrumpe el tratamiento con Zeposia” en la sección 3).
Niños y adolescentes
No administre este medicamento a niños o adolescentes menores de 18 años. Esto se debe a que Zeposia no se ha estudiado en niños ni en adolescentes.
Otros medicamentos y Zeposia
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento. Esto se debe a que Zeposia puede afectar a la forma de funcionar de otros medicamentos. Asimismo, algunos medicamentos pueden afectar a la forma de funcionar de Zeposia.
En particular, antes de tomar Zeposia, informe a su médico o farmacéutico si está tomando o ha tomado recientemente cualquiera de los siguientes medicamentos:
- medicamentos que inhiben o modulan el sistema inmunitario (p. ej., ciclosporina)
- medicamentos utilizados para tratar la EM, como alemtuzumab, interferón beta, dimetil fumarato, acetato de glatiramero, mitoxantrona, natalizumab o teriflunomida
- medicamentos utilizados para tratar la colitis ulcerosa, como azatioprina y 6‑mercaptopurina
- gemfibrozilo para reducir los niveles de grasas o colesterol en la sangre
- clopidogrel, medicamento utilizado para prevenir los coágulos sanguíneos
- rifampicina, un antibiótico para tratar la tuberculosis y otras infecciones graves
- medicamentos llamados inhibidores de la monoaminooxidasa para tratar la depresión (p. ej., fenelzina) o la enfermedad de Parkinson (p. ej., selegilina)
- medicamentos que ralentizan la frecuencia cardiaca (como betabloqueantes o bloqueantes de los canales de calcio)
- cierto tipo de vacunas. Las vacunas vivas atenuadas se deben evitar durante el tratamiento y hasta 3 meses después del mismo.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento.
Embarazo
No utilice Zeposia durante el embarazo, si está intentando quedarse embarazada o si es una mujer con posibilidad de quedarse embarazada y no está utilizando ningún método anticonceptivo efectivo. Si Zeposia se utiliza durante el embarazo, existe el riesgo de dañar al feto. Si es una mujer con posibilidad de quedarse embarazada, su médico le informará sobre los riesgos antes de iniciar el tratamiento con Zeposia y le pedirá que se haga una prueba de embarazo para asegurarse de que no está embarazada. Debe utilizar un método anticonceptivo efectivo mientras está tomando Zeposia y durante al menos 3 meses después de dejar de tomarlo. Pregunte a su médico sobre métodos anticonceptivos fiables.
Su médico le entregará una tarjeta donde se explica por qué no debe quedarse embarazada mientras toma Zeposia.
Si se queda embarazada mientras está tomando Zeposia, informe a su médico inmediatamente. Su médico decidirá interrumpir el tratamiento (ver “Si interrumpe el tratamiento con Zeposia”en la sección 3). Se realizará un control prenatal especializado.
Lactancia
No debe dar el pecho mientras esté tomando Zeposia. Zeposia puede pasar a la leche materna y existe el riesgo de efectos secundarios graves para el bebé.
Conducción y uso de máquinas
La influencia de Zeposia sobre la capacidad para conducir y utilizar máquinas es nula o insignificante.
Zeposia contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por cápsula; esto es, esencialmente “exento de sodio”.
Zeposia contiene potasio
Este medicamento contiene menos de 1 mmol de potasio (39 mg) por cápsula; esto es, esencialmente “exento de potasio”.
3. Cómo tomar Zeposia
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Cuánto se debe tomar
Cuando comienza a tomar Zeposia por primera vez, necesita tomar una dosis baja e ir aumentándola gradualmente para reducir cualquier efecto que ralentice su frecuencia cardiaca.
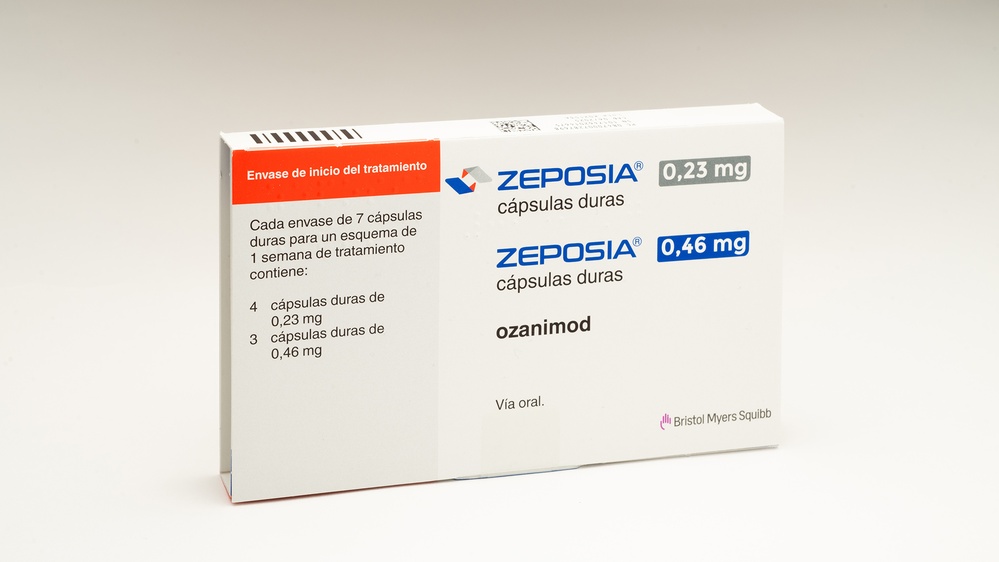
- Usted recibirá un “envase de inicio del tratamiento” para ayudarle a iniciar el tratamiento de esta forma. Contiene:
- 4 cápsulas de color gris claro que contienen 0,23 mg de ozanimod. Tomará una de estas cápsulas los días 1 a 4 del tratamiento.
- 3 cápsulas de color gris claro y naranja que contienen 0,46 mg de ozanimod. Tomará una de estas cápsulas los días 5, 6 y 7.
- A partir del día 8, una vez que haya acabado el “envase de inicio”, continuará con las cápsulas naranjas que contienen la dosis recomendada de 0,92 mg de ozanimod del “envase de mantenimiento”. Continuará el tratamiento regular con una cápsula diaria de 0,92 mg. Si tiene problemas hepáticos crónicos leves o moderados, es posible que su médico tenga que reducirle la dosis de “mantenimiento” a una cápsula de 0,92 mg en días alternos.
Cómo tomar Zeposia
- Zeposia se toma por vía oral.
- Tome la cápsula entera.
- Puede tomar la cápsula con o sin alimentos.
Si toma más Zeposia del que debe
Si toma más Zeposia del que debe, consulte a un médico o acuda a un hospital inmediatamente. Lleve el envase del medicamento y este prospecto con usted.
Si olvidó tomar Zeposia
- Si olvida una o más dosis en los primeros 14 días tras comenzar Zeposia, consulte a su médico sobre cómo reiniciar el tratamiento.
- Si olvida una dosis de Zeposia después de los primeros 14 días tras comenzar Zeposia, tómela en cuanto se acuerde. Sin embargo, si se olvida la dosis durante un día entero, sáltese la dosis olvidada y tome la siguiente dosis a la hora habitual.
- No tome una dosis doble para compensar las dosis olvidadas.
Si interrumpe el tratamiento con Zeposia
- No deje de tomar Zeposia sin consultarlo primero con su médico.
- Consulte a su médico sobre cómo reiniciar el tratamiento si ha dejado de tomar Zeposia:
- durante 1 día o más en los primeros 14 días de tratamiento
- durante más de 7 días consecutivos entre el día 15 y el día 28 de tratamiento
- durante más de 14 días consecutivos después del día 28 de tratamiento.
Tendrá que comenzar de nuevo con el “envase de inicio del tratamiento”.
Zeposia permanecerá en su organismo durante un máximo de 3 meses después de dejar de tomarlo. El recuento de glóbulos blancos (recuento de linfocitos) también puede mantenerse bajo durante este tiempo y todavía pueden producirse los efectos secundarios descritos en este prospecto (ver sección 2 y “Posibles efectos adversos”en la sección 4).
Informe a su médico de inmediato si cree que su EM empeora tras interrumpir el tratamiento con Zeposia.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Efectos adversos graves
Informe a su médico o farmacéutico inmediatamente si observa cualquiera de los efectos adversos graves mencionados a continuación:
- Frecuentes:pueden afectar hasta 1 de cada 10 personas
- frecuencia cardiaca baja
- infección del tracto urinario
- aumento de la tensión arterial.
- Poco frecuentes:pueden afectar hasta 1 de cada 100 personas
- reacción alérgica, los signos pueden incluir una erupción
- visión borrosa (edema macular).
- Raras:pueden afectar hasta 1 de cada 1 000 personas
- infección cerebral denominada leucoencefalopatía multifocal progresiva (LPM) (ver sección 2).
- daño hepático
Otros efectos adversos
Informe a su médico o farmacéutico si observa cualquiera de los siguientes efectos adversos:
- Muy frecuentes:pueden afectar a más de 1 de cada 10 personas
- infecciones de la nariz o las fosas nasales, la cavidad nasal, la boca, la garganta (faringe) o la laringe causada por virus
- nivel bajo de un tipo de glóbulos blancos llamados linfocitos.
- Frecuentes:pueden afectar hasta 1 de cada 10 personas
- inflamación de la garganta (faringitis)
- infección respiratoria provocada por un virus (signo de infección pulmonar)
- herpes zóster (culebrilla)
- herpes simple o calenturas bucales (herpes labial)
- dolor de cabeza
- bajada de la tensión arterial
- hinchazón especialmente de tobillos y de pies, debido a la retención de líquidos (edema periférico)
- aumento de los niveles de las enzimas hepáticas o de la bilirrubina en los análisis de sangre (un signo de problemas hepáticos) o pigmentación amarillenta en la piel, mucosa y ojos, hiperbilirrubinemia o niveles altos de bilirrubina en sangre (ictericia)
- anomalías pulmonares que pueden causar falta de aliento.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Zeposia
- Mantener este medicamento fuera de la vista y del alcance de los niños.
- No utilice este medicamento después de la fecha de caducidad que aparece en el blíster y en la caja después de “CAD”/“EXP”. La fecha de caducidad es el último día del mes que se indica.
- No conservar a temperatura superior a 25°C.
- No utilice este medicamento si observa algún daño o indicios de manipulación indebida en el envase.
- Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Zeposia
- El principio activo es ozanimod.
- Zeposia 0,23mg cápsulas duras
Cada cápsula dura contiene 0,23 mg de ozanimod (como clorhidrato).
- Zeposia 0,46mg cápsulas duras
Cada cápsula dura contiene 0,46 mg de ozanimod (como clorhidrato).
- Zeposia 0,92mg cápsulas duras
Cada cápsula dura contiene 0,92 mg de ozanimod (como clorhidrato).
- Los demás componentes son
- Contenido de la cápsula:
Celulosa microcristalina, sílice coloidal anhidra, croscarmelosa sódica y estearato de magnesio.
- Cubierta de la cápsula:
- Cada cápsula de 0,23 mg contiene gelatina, dióxido de titanio (E171), óxido de hierro amarillo (E172), óxido de hierro negro (E172) y óxido de hierro rojo (E172).
- Cada cápsula de 0,46 mg contiene gelatina, dióxido de titanio (E171), óxido de hierro amarillo (E172), óxido de hierro negro (E172) y óxido de hierro rojo (E172).
- Cada cápsula de 0,92 mg contiene gelatina, dióxido de titanio (E171), óxido de hierro amarillo (E172) y óxido de hierro rojo (E172).
- Tinta de impresión:óxido de hierro negro (E172), goma laca (E904), polipropilenglicol (E1520), solución de amoniaco concentrado (E527) e hidróxido de potasio (E525).
Aspecto del producto y contenido del envase
- La cápsula dura de Zeposia 0,23 mg de 14,3 mm tiene una tapa y un cuerpo de color gris claro opaco, con la inscripción “OZA” en la tapa y “0.23 mg” en el cuerpo en tinta negra.
- La cápsula dura de Zeposia 0,46 mg de14,3 mm tiene una tapa de color naranja opaco y un cuerpo de color gris claro opaco, con la inscripción “OZA” en la tapa y “0.46 mg” en el cuerpo en tinta negra.
- La cápsula dura de Zeposia 0,92 mg de 14,3 mm tiene una tapa y un cuerpo de color naranja opaco, con la inscripción “OZA” en la tapa y “0.92 mg” en el cuerpo en tinta negra.
Tamaños de envase
- El envase de inicio del tratamiento es un estuche que contiene 7 cápsulas duras: 4 cápsulas duras de 0,23 mg y 3 cápsulas duras de 0,46 mg.
- El envase de mantenimiento contiene 28 cápsulas duras de 0,92 mg o 98 cápsulas duras de 0,92 mg.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Bristol‑Myers Squibb Pharma EEIG
Plaza 254
Blanchardstown Corporate Park 2
Dublin 15, D15 T867
Irlanda
Responsable de la fabricación
Celgene Distribution B.V.
Orteliuslaan 1000
3528 BD Utrecht
Países Bajos
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien N.V. Bristol‑Myers Squibb Belgium S.A. Tél/Tel: + 32 2 352 76 11 | Lietuva Swixx Biopharma UAB Tel: + 370 52 369140 |
| Luxembourg/Luxemburg N.V. Bristol‑Myers Squibb Belgium S.A. Tél/Tel: + 32 2 352 76 11 |
Ceská republika Bristol‑Myers Squibb spol. s r.o. Tel: + 420 221 016 111 | Magyarország Bristol‑Myers Squibb Kft. Tel.: + 36 1 301 9797 |
Danmark Bristol‑Myers Squibb Denmark Tlf: + 45 45 93 05 06 | Malta A.M. Mangion Ltd Tel: + 356 23976333 |
Deutschland Bristol‑Myers Squibb GmbH & Co. KGaA Tel: 0800 0752002 (+ 49 89 121 42 350) | Nederland Bristol‑Myers Squibb B.V. Tel: + 31 (0)30 300 2222 |
Eesti Swixx Biopharma OÜ Tel: + 372 640 1030 | Norge Bristol‑Myers Squibb Norway AS Tlf: + 47 67 55 53 50 |
Ελλáδα Bristol‑Myers Squibb A.E. Τηλ: + 30 210 6074300 | Österreich Bristol‑Myers Squibb GesmbH Tel: + 43 1 60 14 30 |
España Bristol‑Myers Squibb, S.A. Tel: + 34 91 456 53 00 | Polska Bristol‑Myers Squibb Polska Sp. z o.o. Tel.: + 48 22 2606400 |
France Bristol‑Myers Squibb SAS Tél: + 33 (0)1 58 83 84 96 | Portugal Bristol‑Myers Squibb Farmacêutica Portuguesa, S.A. Tel: + 351 21 440 70 00 |
Hrvatska Swixx Biopharma d.o.o. Tel: + 385 1 2078 500 | România Bristol‑Myers Squibb Marketing Services S.R.L. Tel: + 40 (0)21 272 16 19 |
Ireland Bristol‑Myers Squibb Pharmaceuticals uc Tel: 1 800 749 749 (+ 353 (0)1 483 3625) | Slovenija Swixx Biopharma d.o.o. Tel: + 386 1 2355 100 |
Ísland Vistor ehf. Sími: + 354 535 7000 | Slovenská republika Swixx Biopharma s.r.o. Tel: + 421 2 20833 600 |
Italia Bristol‑Myers Squibb S.r.l. Tel: + 39 06 50 39 61 | Suomi/Finland Oy Bristol‑Myers Squibb (Finland) Ab Puh/Tel: + 358 9 251 21 230 |
Κúπρος Bristol‑Myers Squibb A.E. Τηλ: 800 92666 (+ 30 210 6074300) | Sverige Bristol‑Myers Squibb Aktiebolag Tel: + 46 8 704 71 00 |
Latvija Swixx Biopharma SIA Tel: + 371 66164750 |
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: https://www.ema.europa.eu/.
Puede acceder a información detallada sobre este medicamento escaneando con su teléfono móvil (smartphone) el código QR incluido en el embalaje exterior. También puede acceder a esta información en la siguiente dirección de internet: www.zeposia-eu-pil.com.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ZEPOSIA 0,23 MG/0,46 MG CAPSULAS DURASForma farmacéutica: CAPSULA, 0,92 mgPrincipio activo: ozanimodFabricante: Bristol-Myers Squibb Pharma EeigRequiere recetaForma farmacéutica: CAPSULA, 0,5 mgPrincipio activo: FingolimodFabricante: Accord Healthcare S.L.U.Requiere recetaForma farmacéutica: CAPSULA, 0.5 mgPrincipio activo: FingolimodFabricante: Aurovitas Spain, S.A.U.Requiere receta
Médicos online para ZEPOSIA 0,23 MG/0,46 MG CAPSULAS DURAS
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ZEPOSIA 0,23 MG/0,46 MG CAPSULAS DURAS, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes







