
VUEWAY 0,5 MMOL/ML SOLUCION INYECTABLE

Cómo usar VUEWAY 0,5 MMOL/ML SOLUCION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
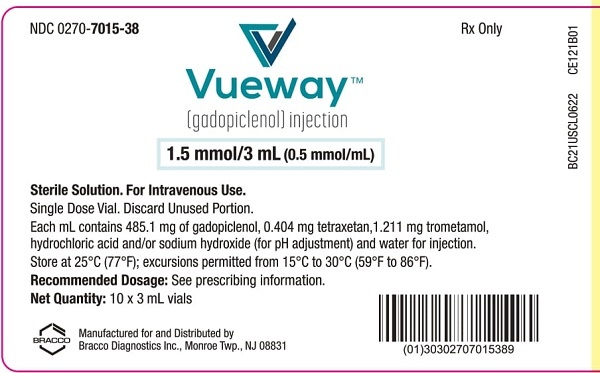
Vueway 0,5 mmol/ml solución inyectable
gadopiclenol
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de que le administren este medicamento porque contiene información importante para usted.
- Conserve este prospecto, ya que puede que tenga que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, radiólogo o farmacéutico.
- Si experimenta efectos adversos, consulte a su médico o radiólogo, incluso si no aparece en este prospecto. Ver sección 4.
Contenido de este prospecto
- Qué es Vueway y para qué se utiliza
- Qué necesita saber antes de que se le administre Vueway
- Cómo se le administrará Vueway
- Posibles efectos adversos
- Conservación de Vueway
- Contenido del envase e información adicional
1. Qué es Vueway y para qué se utiliza
Vueway es un medio de contraste que mejora el contraste de las imágenes obtenidas durante las exploraciones de resonancia magnética (RM). Vueway contiene el principio activo gadopiclenol.
Mejora la visualización y delineación de estructuras anómalas o lesiones de determinadas partes del cuerpo y ayuda a diferenciar entre tejido sano y enfermo.
Se utiliza en adultos y niños (a partir de 2 años de edad).
Se administra en forma de inyección en vena. Este medicamento es sólo para uso diagnóstico y sólo será administrado por profesionales sanitarios con experiencia en la práctica clínica de la resonancia magnética.
2. Qué necesita saber antes de que se le administre Vueway
Vueway no se debe administrar
si se es alérgico al principio activo o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Consulte a su médico, radiólogo o farmacéutico antes de que le administren Vueway:
- si ha tenido una reacción previa a cualquier agente de contraste,
- si tiene asma,
- si tiene antecedentes de alergia (tales como, fiebre del heno, urticaria),
- si sus riñones no funcionan correctamente,
- si ha sufrido convulsiones o está en tratamiento por epilepsia,
- si tiene alguna enfermedad del corazón o los vasos sanguíneos,
En todos estos casos, su médico decidirá si el examen previsto es posible o no. Si se le administra Vueway, su médico o radiólogo debe tomar las precauciones necesarias y administrarlo bajo estrecha vigilancia.
Su médico o radiólogo puede decidir realizarle un análisis de sangre para comprobar el correcto funcionamiento de sus riñones antes de decidir el uso de Vueway, especialmente si usted tiene 65 años de edad o es mayor.
Otros medicamentos y Vueway
Informe a su médico, radiólogo o farmacéutico si está tomando, ha tomado recientemente o puede tener que tomar otros medicamentos.
En particular, informe a su médico, radiólogo o farmacéutico si está recibiendo o ha recibido recientemente medicamentos para tratar el corazón o la presión sanguínea como betabloqueantes, sustancias vasoactivas, inhibidores de la enzima convertidora de la angiotensina (ACE) o antagonistas del receptor de la angiotensina II.
Embarazo y lactancia
Embarazo
Gadopiclenol puede atravesar la placenta. No se sabe si afecta al bebé. Consulte a su médico o radiólogo, si está embarazada o si cree que podría estar embarazada, ya que Vueway no debe utilizarse durante el embarazo a menos que sea estrictamente necesario.
Lactancia
Consulte a su médico o radiólogo si está en período de lactancia.
Su médico valorará si debe continuar la lactancia o si debe interrumpirla hasta 24 horas después de recibir Vueway.
Conducción y uso de máquinas
Vueway tiene un efecto nulo o insignificante sobre la capacidad para conducir y utilizar máquinas. Sin embargo, si se siente indispuesto después de la exploración, no debe conducir o usar máquinas.
Vueway contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por vial de 15 ml; esto es, esencialmente "exento de sodio".
3. Cómo se le administrará Vueway
Un profesional sanitario especializado le inyectará Vueway en la vena con una pequeña aguja. Vueway puede inyectarse manualmente o con un inyector automático.
Su médico o radiólogo determinará la dosis que debe recibir y supervisará la inyección.
La dosis habitual de 0,1 ml/kg de peso corporal es la misma en adultos y niños a partir de 2 años de edad.
En los niños, su médico o radiólogo utilizará Vueway en viales con una jeringa de un solo uso para poder tener una mayor precisión del volumen inyectado.
Tras la inyección, permanecerá bajo supervisión durante al menos 30 minutos. Este es el momento en el que pueden producirse más reacciones no deseadas (tales como reacciones alérgicas). Sin embargo, en raras ocasiones, pueden producirse reacciones al cabo de horas o días.
Uso en pacientes con problemas renales graves
No se recomienda el uso de Vueway en pacientes con problemas renales graves. Sin embargo, si se requiere su uso, solo debe administrarse una única dosis durante la exploración y no administrar una segunda inyección hasta que hayan transcurrido al menos 7 días.
Uso en pacientes de edad avanzada
Si usted tiene 65 años de edad o más, no es necesario que se le ajuste la dosis, pero podría realizársele un análisis de sangre para comprobar el correcto funcionamiento de sus riñones.
Si recibe más Vueway del que debe
Es muy poco probable que reciba una sobredosis de Vueway, ya que se lo administrará un profesional sanitario cualificado. Si se produce, Vueway puede eliminarse del organismo mediante hemodiálisis (limpieza de sangre).
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, radiólogo o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Tras la administración de Vueway, permanecerá en observación. La mayoría de los efectos adversos se produce en minutos. Existe un pequeño riesgo de sufrir una reacción alérgica a Vueway. Estos efectos pueden producirse inmediatamente o hasta siete días después de la inyección. Estas reacciones pueden ser graves y causar un shock (caso de reacción alérgica que puede poner su vida en peligro).
Informe inmediatamente a su médico, radiólogo o profesional sanitario si sufre alguno de los siguientes efectos adversos, ya que pueden ser los primeros signos de un shock:
- hinchazón de la cara, labios, lengua o garganta
- mareo (presión arterial baja)
- dificultad respiratoria
- erupción cutánea
- tos, estornudos o secreción nasal
Los posibles efectos secundarios que se han observado durante los ensayos clínicos con Vueway se enumeran a continuación en función de su probabilidad:
Frecuencia | Posibles efectos adversos |
Frecuentes(pueden afectar hasta 1 de cada 10 personas) | Reacción en el lugar de inyección* Cefalea |
Nada frecuentes (pueden afectar hasta 1 de cada 100 personas) | Reacción alérgica** Diarrea Náuseas (sensación de malestar) Fatiga (cansancio) Dolor abdominal Sabor inusual en la boca Sensación de calor Vómitos (estar indispuesto) |
*La reacción en el lugar de inyección puede ser dolor, hinchazón, sensación de frío o de calor, hematoma y enrojecimiento.
**Las reacciones alérgicas pueden ser: inflamación de la piel, enrojecimiento de la piel, dificultad respiratoria, alteración de la voz, opresión en la garganta, irritación de la garganta, sensación anormal en la boca, enrojecimiento transitorio de la cara (reacciones tempranas) y ojos hinchados, hinchazón, erupción cutánea y picor (reacciones tardías).
Se han notificado casos de fibrosis nefrogénica sistémica (FNS) (que provoca un endurecimiento de la piel y puede afectar también a los tejidos blandos y órganos internos) con otros medios de contraste quecontienen gadolinio, pero no se ha notificado ningún caso de FNS con Vueway durante los ensayos clínicos.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es
Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento
5. Conservación de Vueway
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta del vial o de la jeringa precargada y en la caja de cartón después de "EXP" o “CAD”. La fecha de caducidad se refiere al último día de ese mes.
Este medicamento es una solución transparente, entre incolora y amarillo pálido.
No utilice este medicamento si la solución no es transparente o si contiene partículas visibles.
Para viales: Este medicamento no requiere condiciones especiales de conservación.
Se ha demostrado la estabilidad química y física durante 24 horas a una temperatura de hasta 25 °C.
Desde el punto de vista microbiológico, el producto debe utilizarse inmediatamente después de abrirlo.
Para jeringas precargadas: No congelar.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Vueway
- El principio activo es gadopiclenol. Cada ml de solución contiene 485,1 mg de gadopiclenol (equivalente a 0,5 mmol de gadopiclenol y a 78,6 mg de gadolinio).
- Los demás componentes son tetraxetan, trometamol, ácido clorhídrico (para ajustar el pH), hidróxido de sodio (para ajustar el pH) y agua para preparaciones inyectables. Ver sección 2 “Vueway contiene sodio”.
Aspecto de Vueway y contenido del envase
Es una solución inyectable transparente, entre incolora y amarillo pálido.
Está disponible en envases que incluyen:
- 1 vial con 3, 7,5, 10, 15, 30, 50 o 100 ml de solución inyectable.
- 25 viales con 7,5, 10 o 15 ml de solución inyectable.
- 1 o 10 (10 x 1) jeringas precargadas con 7,5, 10 o 15 ml de solución inyectable.
- 1 jeringa precargada con 7,5, 10 o 15 ml de solución inyectable con kit de administración para inyección manual (una línea de extensión y un catéter).
- 1 jeringa precargada con 7,5, 10 o 15 ml de solución inyectable con kit de administración para inyector Optistar Elite (una línea de extensión, un catéter y una jeringa vacía de plástico de 60 ml).
- 1 jeringa precargada con 7,5, 10 o 15 ml de solución inyectable con kit de administración para inyector Medrad Spectris Solaris EP (una extensión de línea, un catéter y una jeringa vacía de plástico de 115 ml).
Puede que solamente estén comercializados algunos tamaños de envase.
Titular de la autorización de comercialización
Bracco Imaging SPA
Via Egidio Folli, 50
20134 Milano
Italia
Fabricante
Guerbet
16 rue Jean Chaptal
93600 Aulnay-sous-Bois
Francia
BIPSO GmbH
Robert-Gerwig-Strasse 4
Singen (Hohentwiel)
78224
Alemania
Fecha de la última revisión de este prospecto diciembre 2024
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
------------------------------------------------------------------------------------------------------------------
Esta información está destinada únicamente a profesionales sanitarios:
Para más detalles sobre cómo utilizar el medicamento, consulte la sección 6.6 Precauciones especiales de eliminación y otras manipulaciones de la Ficha técnica de este medicamento.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a VUEWAY 0,5 MMOL/ML SOLUCION INYECTABLEForma farmacéutica: INYECTABLE, 0,5 mmol/mlPrincipio activo: GadopiclenolFabricante: GuerbetRequiere recetaForma farmacéutica: INYECTABLE, 0,5 mmol/mlPrincipio activo: GadopiclenolFabricante: GuerbetRequiere recetaForma farmacéutica: INYECTABLE, 0,5 mmol/mlPrincipio activo: Gadoterico acidoFabricante: Ge Healthcare Bio-Sciences, S.A.U.Requiere receta
Médicos online para VUEWAY 0,5 MMOL/ML SOLUCION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de VUEWAY 0,5 MMOL/ML SOLUCION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















