
VEROLAX ADULTOS RECTAL SOLUTION

How to use VEROLAX ADULTOS RECTAL SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
VEROLAX ADULTS Rectal Solution
Glycerol
Read this leaflet carefully because it contains important information for you.
This medication can be obtained without a prescription. Nevertheless, to obtain the best results, it should be used properly.
- Keep this leaflet, as you may need to read it again.
- If you need advice or more information, consult your pharmacist.
- If symptoms worsen or persist after 7 days, you should consult a doctor.
- If you consider that any of the adverse effects you are suffering from is serious or if you notice any adverse effect not mentioned in this leaflet, inform your doctor or pharmacist.
Contents of the Package Leaflet
- What is VEROLAX and what is it used for
- Before using VEROLAX
- How to use VEROLAX
- Possible side effects
- Storage of VEROLAX
- Additional information
1. What is VEROLAX and what is it used for
Glycerol, the active ingredient of this medication, is a laxative that is administered rectally. The laxative effect is achieved by the ability of glycerol to soften the stools, which, together with a mild local irritating action, stimulates the movements of the intestine.
It is indicated for the local symptomatic relief of transient and occasional constipation in adults and adolescents from 12 years of age.
2. Before using VEROLAX
- If you are allergic (hypersensitive) to glycerol or any of the other components of this medication.
- If you suffer from any anorectal disease, rectocolitis hemorrhagica (a type of chronic inflammation of the intestine), and inflamed hemorrhoids.
- If you have colic, nausea, vomiting, or other signs of appendicitis, intestinal obstruction, acute inflammatory intestinal diseases, or any situation of abdominal pain, without knowing the cause that produces them.
- Children under 12 years of age.
Be careful with VEROLAX
In case of the appearance of blood in stools, irritation, pain, or no improvement, you should interrupt treatment and consult a doctor.
Do not use this medication for more than 7 consecutive days, unless your doctor indicates otherwise.
This medication will be used only under strict medical control in patients with severe diseases, especially cardiovascular (related to the heart or blood vessels).
Use of other medications
Inform your doctor or pharmacist if you are using or have recently used other medications, including those purchased without a prescription.
Use in children
Do not administer to children under 12 years of age.
Pregnancy and breastfeeding
Consult your doctor or pharmacist before using any medication.
Driving and using machines
The use of this medication does not affect the ability to drive and/or use machines.
3. How to use VEROLAX
Follow exactly the administration instructions of VEROLAX indicated by your doctor.
Consult your doctor or pharmacist if you have doubts.
The normal dose is:
Adults and adolescents over 12 years: 1 single-dose container per day.
This medication is administered rectally.
Instructions for the correct administration of the medication:
- Open the applicator by holding it by the neck and twisting the cap, facilitating the breaking of the seal.
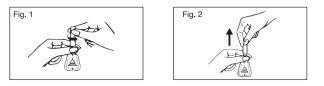
- Slightly press to humidify the tip of the cannula with a few drops of the medication, in order to facilitate its introduction into the rectum.
- Introduce the cannula of the applicator into the rectum smoothly and slowly.
- Introduce the contents of the applicator by pressing on the walls of the bellows and without stopping pressing, gently remove it once the application is finished.
Each applicator is loaded with an excess of product, which remains in the applicator after use, so that the amount released in the application is the complete dose and it is not necessary to empty it completely, which facilitates its handling. It should be discarded after use.
If you notice resistance during administration, you should interrupt it, as it may be harmful and damaging.
Do not use the medication for more than 7 consecutive days. If symptoms do not improve, you should suspend treatment and consult a doctor.
If you use more VEROLAX than you should
It is unlikely that intoxication will occur due to its use.
The abusive and prolonged use of this medication can lead to an irritable colon syndrome (symptoms or discomfort such as alternation of constipation and diarrhea, intestinal spasms, bloating, nausea, and gases).
In case of overdose or accidental ingestion, consult your doctor, pharmacist, or call the Toxicology Information Service, phone 91.562.04.20, indicating the medication and the amount ingested.
4. Possible side effects
Like all medications, VEROLAX can produce side effects, although not all people suffer from them.
During the period of use of VEROLAX rectal solution as a laxative, the following side effects have been observed, whose frequency has not been established with precision:
itching, pain, and irritation of the anus.
Very rarely, in allergic patients, hypersensitivity reactions to chamomile (e.g., contact dermatitis) have occurred. After internal use, these could be serious and could be: swelling of the face, lips, mouth, tongue, or throat that causes difficulty swallowing or breathing.
If you consider that any of the side effects you are suffering from is serious or if you notice any side effect not mentioned in this leaflet, inform your doctor or pharmacist.
5. Storage of VEROLAX
Keep out of the reach and sight of children.
No special storage conditions are required.
Do not use VEROLAX after the expiration date that appears on the container, after CAD. The expiration date is the last day of the month indicated.
Medications should not be thrown away by drains or in the trash. Ask your pharmacist how to dispose of the containers and medications that you no longer need. This way, you will help protect the environment.
6. ADDITIONAL INFORMATION
Composition of VEROLAX
- The active ingredient is glycerol. Each single-dose container with 7.5 ml of rectal solution contains 5.4 ml of glycerol.
- The other components (excipients) are: fluid extract of Malva sylvestris, fluid extract of Matricaria chamomilla, wheat starch, and purified water.
Appearance of the product and contents of the container
VEROLAX is presented as a rectal solution of light brown color.
The medication is packaged in a single-dose container in the form of a 7.5 ml bellows, provided with a plastic cannula and a closing cap. These are packaged in a box that contains 6 single-dose containers.
Marketing authorization holder
ANGELINI PHARMA ESPAÑA, S.L.
c/ Antonio Machado, 78-80
3rd floor, module A-Australia Building
08840 Viladecans, Barcelona (Spain)
Manufacturer
A.C.R.A.F. SpA
Via Vecchia del Pinocchio, 22
60131 Ancona (Italy)
This leaflet was approved in May 2011.
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to VEROLAX ADULTOS RECTAL SOLUTIONDosage form: RECTAL LIQUID, 6.14 ml glycerolActive substance: glycerolManufacturer: Casen Recordati S.L.Prescription not requiredDosage form: RECTAL LIQUID, 6.75 g/single-dose tubeActive substance: glycerolManufacturer: Laboratorios Cinfa S.A.Prescription not requiredDosage form: RECTAL LIQUID, 5.4 ml of glycerol/single-dose containerActive substance: glycerolManufacturer: Lainco S.A.Prescription not required
Online doctors for VEROLAX ADULTOS RECTAL SOLUTION
Discuss questions about VEROLAX ADULTOS RECTAL SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















