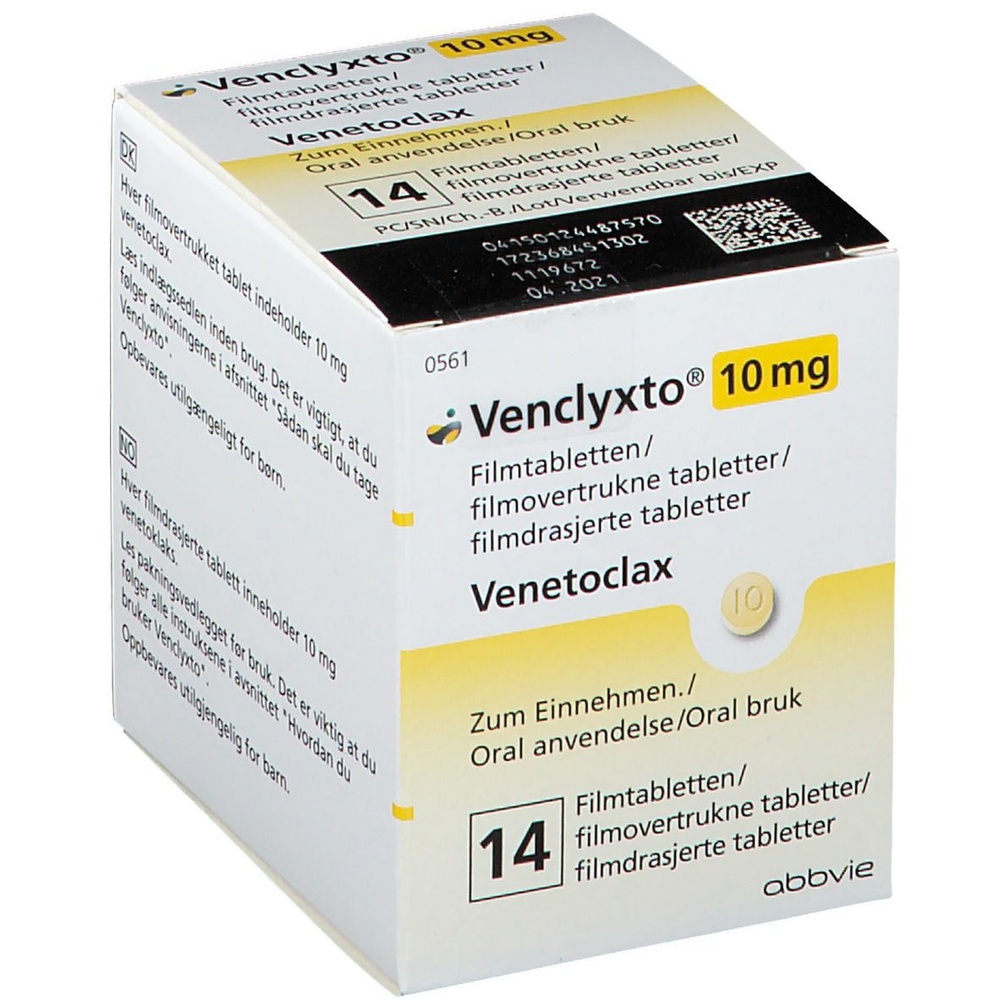
VENCLYXTO 10 MG COMPRIMIDOS RECUBIERTOS CON PELICULA

Cómo usar VENCLYXTO 10 MG COMPRIMIDOS RECUBIERTOS CON PELICULA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Venclyxto 10mg comprimidos recubiertos con película
Venclyxto 50mg comprimidos recubiertos con película
Venclyxto 100mg comprimidos recubiertos con película
venetoclax
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Venclyxto y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Venclyxto
- Cómo tomar Venclyxto
- Posibles efectos adversos
- Conservación de Venclyxto
- Contenido del envase e información adicional
1. Qué es Venclyxto y para qué se utiliza
Qué es Venclyxto
Venclyxto es un medicamento para el cáncer que contiene el principio activo venetoclax. Pertenece a un grupo de medicamentos denominado “inhibidores de BCL-2”.
Para qué se utiliza Venclyxto
Venclyxto se utiliza para tratar a adultos con:
- leucemia linfocítica crónica (LLC). Es posible que reciba Venclyxto como tratamiento único o en combinación con otros medicamentos.
- leucemia mieloide aguda (LMA). Recibirá Venclyxto en combinación con otros medicamentos.
La LLC es un tipo de cáncer que afecta a un tipo de glóbulos blancos, llamados linfocitos, y a los ganglios linfáticos. En la LLC, los linfocitos se multiplican demasiado deprisa y viven demasiado tiempo, de manera que existe un exceso de estos en la sangre.
La LMA es un tipo de cáncer que afecta a un tipo de glóbulos blancos llamados células mieloides. En la LMA, las células mieloides se multiplican y crecen muy rápidamente en la médula ósea y en la sangre, por lo que hay demasiada cantidad de ellas y una cantidad no suficiente de glóbulos rojos en la sangre.
Cómo funciona Venclyxto
Venclyxto actúa bloqueando una proteína del organismo llamada “BCL-2”. Esta proteína está presente en altas cantidades en algunas células cancerosas y ayuda a las células cancerosas a sobrevivir. El bloqueo de esta proteína facilita la destrucción de las células cancerosas y la reducción de su número. También retrasa el empeoramiento de la enfermedad
2. Qué necesita saber antes de empezar a tomar Venclyxto
No tome Venclyxto si:
- es alérgico al principio activo venetoclax o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- tiene LLC y está tomando alguno de los medicamentos que figuran a continuación al empezar el tratamiento y durante el incremento gradual de la dosis (generalmente durante 5 semanas). No debe hacerlo porque pueden producirse efectos graves y potencialmente mortales al tomar Venclyxto con estos medicamentos:
- itraconazol, ketoconazol, posaconazol o voriconazol para las infecciones por hongos.
- claritromicina para las infecciones bacterianas.
- ritonavir para la infección por el VIH.
Una vez que la dosis de Venclyxto se haya aumentado hasta la dosis estándar completa, consulte con su médico si puede comenzar a tomar estos medicamentos otra vez.
- está tomando un medicamento a base de plantas llamado hierba de San Juan, utilizado para la depresión. Si no está seguro, hable con su médico, farmacéutico o enfermero antes de tomar Venclyxto.
Es importante que informe a su médico, farmacéutico o enfermero acerca de todos los medicamentos que toma, incluidos los de venta con y sin receta médica, las vitaminas y complementos a base de plantas. Es posible que el médico le pida que deje de tomar determinados medicamentos cuando empiece a tomar Venclyxto y durante los primeros días o semanas, durante el aumento de la dosis hasta la dosis estándar completa.
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de empezar a tomar Venclyxto si:
- tiene algún problema de riñón, ya que puede aumentar el riesgo de sufrir un efecto adverso llamado síndrome de lisis tumoral.
- tiene problemas de hígado, ya que puede aumentar el riesgo de sufrir efectos adversos. Su médico podría tener que reducir su dosis de Venclyxto.
- cree que podría tener una infección o ha tenido una infección de larga duración o recurrente.
- tiene cita para ponerse una vacuna.
Si se encuentra en alguna de las circunstancias anteriores, o no está seguro, consulte a su médico, farmacéutico o enfermero antes de tomar este medicamento.
Síndrome de Lisis Tumoral
Algunas personas pueden presentar una cantidad inusual de ciertas sales del organismo en la sangre (tales como potasio y ácido úrico), a consecuencia de la degradación rápida de las células del cáncer durante el tratamiento. Esta situación puede provocar alteraciones en el funcionamiento de los riñones, un ritmo cardíaco anormal o convulsiones. La afección se denomina síndrome de lisis tumoral (SLT). El riesgo de SLT se da en los primeros días o semanas de tratamiento con Venclyxto, cuando se aumenta la dosis.
Si tiene LLC
Su médico, farmacéutico o enfermero le hará análisis de sangre para detectar la presencia de SLT.
Antes de empezar el tratamiento con Venclyxto, su médico le administrará medicamentos para ayudar a prevenir la acumulación de ácido úrico en el organismo.
Beber mucha agua, al menos 1,5 a 2 litros al día, ayuda a eliminar del cuerpo los productos de desecho de las células del cáncer a través de la orina y puede disminuir el riesgo de sufrir un SLT (ver la sección 3).
Informe a su médico, farmacéutico o enfermero de inmediato si tiene alguno de los síntomas de SLT que figuran en la sección 4.
Si está en riesgo de SLT, es posible que reciba el tratamiento en el hospital para poder administrarle líquidos intravenosos si fuera necesario, hacerle análisis de sangre con más frecuencia y revisar si hay efectos adversos. Esta precaución se toma para saber si puede seguir tomando este medicamento de manera segura.
Si tiene LMA
Puede recibir el tratamiento en el hospital y su médico o enfermero se asegurarán de que tenga suficiente agua o líquidos, le administrarán medicamentos para prevenir la acumulación de ácido úrico en su organismo y le realizarán análisis de sangre antes de empezar a tomar Venclyxto, mientras van aumentando la dosis y cuando empiece a tomar la dosis completa.
Niños y adolescentes
Venclyxto no se debe usar en niños y adolescentes.
Otros medicamentos y Venclyxto
Informe a su médico o farmacéutico si está tomando alguno de los siguientes medicamentos, ya que estos pueden aumentar o reducir la cantidad de venetoclax en la sangre:
- medicamentos para las infecciones por hongos - fluconazol, itraconazol, ketoconazol, posaconazol o voriconazol
- antibióticos para las infecciones bacterianas - ciprofloxacino, claritromicina, eritromicina, nafcilina o rifampicina
- medicamentos para prevenir las convulsiones o para tratar la epilepsia - carbamazepina, fenitoína
- medicamentos para la infección por el VIH - efavirenz, etravirina, ritonavir
- medicamentos para tratar la hipertensión arterial o la angina de pecho - diltiazem, verapamilo
- medicamentos para reducir los niveles de colesterol en sangre – colestiramina, colestipol, colesevelam
- un medicamento utilizado para tratar una alteración pulmonar denominada hipertensión arterial pulmonar - bosentán
- un medicamento para tratar un trastorno del sueño (narcolepsia) llamado modafinilo
- un medicamento a base de plantas conocido como hierba de San Juan
Es posible que su médico le cambie la dosis de Venclyxto.
Informe a su médico si está tomando alguno de los siguientes medicamentos, ya que Venclyxto puede afectar a su funcionamiento:
- medicamentos que previenen coágulos, warfarina, dabigatrán
- un medicamento para tratar problemas cardíacos, llamado digoxina
- un medicamento contra el cáncer denominado everolimus
- un medicamento para prevenir el rechazo de órganos conocido como sirolimus
- medicamentos para disminuir los niveles de colesterol en sangre conocidos como estatinas
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento. Incluidos los medicamentos obtenidos sin receta, los medicamentos a base de plantas y los complementos. Debe hacerlo porque Venclyxto puede afectar a la forma en que funcionan algunos medicamentos. Además, otros medicamentos pueden afectar a la forma en que funciona Venclyxto.
Toma de Venclyxto con alimentos y bebidas
No tome productos con pomelo, naranjas de Sevilla (naranjas amargas, frecuentemente usadas en mermeladas) ni carambola (fruta estrella) mientras esté tomando Venclyxto - no debe comerlos, beber su zumo ni tomar complementos que pudieran contenerlos. El motivo es que pueden aumentar la cantidad de venetoclax en la sangre.
Embarazo
- No se quede embarazada mientras esté tomando este medicamento. Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico, farmacéutico o enfermero antes de utilizar este medicamento.
- Venclyxto no se debe utilizar durante el embarazo. No hay información sobre la seguridad de venetoclax en mujeres embarazadas.
Anticoncepción
- Las mujeres en edad fértil tienen que utilizar un método anticonceptivo altamente eficaz durante el tratamiento y al menos 30 días después de haber recibido Venclyxto para evitar quedarse embarazadas. Si está utilizando píldoras o dispositivos anticonceptivos hormonales, tiene que utilizar también un método anticonceptivo de barrera (como el preservativo) porque el efecto de los anticonceptivos hormonales, en píldora o dispositivo, puede verse afectado por Venclyxto.
- Informe inmediatamente a su médico si se queda embarazada mientras está tomando este medicamento.
Lactancia
No amamante a su hijo mientras esté tomando este medicamento. Se desconoce si el principio activo de Venclyxto pasa a la leche materna.
Fertilidad
Sobre la base de los resultados obtenidos en estudios en animales, Venclyxto puede causar infertilidad masculina (cantidad de espermatozoides escasa o nula). Ello puede afectar a su capacidad para tener un hijo. Consulte a su médico sobre almacenamiento de esperma antes de empezar el tratamiento con Venclyxto.
Conducción y uso de máquinas
Después de tomar Venclyxto, es posible que se sienta cansado o mareado, lo cual puede afectar a su capacidad para conducir y utilizar herramientas o máquinas. Si esto sucede, no conduzca o use ninguna herramienta o máquina.
Venclyxto contiene sodio.
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por dosis; esto es, esencialmente “exento de sodio”.
3. Cómo tomar Venclyxto
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico, farmacéutico o enfermero. En caso de duda, consulte de nuevo a su médico, farmacéutico o enfermero.
Cantidad que debe tomar
Si tiene LLC
Empezará el tratamiento con Venclyxto a una dosis baja durante 1 semana. El médico le aumentará gradualmente la dosis durante las próximas 4 semanas hasta la dosis estándar completa. Durante las primeras 4 semanas, recibirá un nuevo envase cada semana.
- la dosis inicial es de 20 mg (dos comprimidos de 10 mg) una vez al día durante 7 días.
- la dosis se aumentará a 50 mg (un comprimido de 50 mg) una vez al día durante 7 días.
- la dosis se aumentará a 100 mg (un comprimido de 100 mg) una vez al día durante 7 días.
- la dosis se aumentará a 200 mg (dos comprimidos de 100 mg) una vez al día durante 7 días.
- la dosis se aumentará a 400 mg (cuatro comprimidos de 100 mg) una vez al día durante 7 días.
- Si está recibiendo Venclyxto como tratamiento único, seguirá tomando la dosis diaria de 400 mg, que es la dosis diaria recomendada, durante el tiempo necesario.
- Si está recibiendo Venclyxto en combinación con rituximab, tomará la dosis diaria de 400 mg durante 24 meses.
- Si está recibiendo Venclyxto en combinación con obinutuzumab, tomará la dosis diaria de 400 mg durante 10 meses aproximadamente.
Es posible que sea necesario un ajuste de dosis debido a los efectos adversos. Su médico le indicará qué dosis debe tomar.
Si tiene LMA
Empezará el tratamiento con Venclyxto a una dosis más baja. Su médico irá aumentando la dosis gradualmente cada día durante los primeros 3 días. Transcurridos los 3 días, tomará la dosis estándar completa. La dosis (comprimidos) se toma una vez al día.
En la siguiente tabla se indican las dosis
Día | Dosis diaria de Venclyxto |
1 | 100 mg (un comprimido de 100 mg) |
2 | 200 mg (dos comprimidos de 100 mg) |
3 y posteriores | 400 mg (cuatro comprimidos de 100 mg) |
Su médico le administrará Venclyxto en combinación con otro medicamento (azacitidina o decitabina).
Continuará tomando la dosis completa de Venclyxto hasta que empeore la LMA o no pueda tomar Venclyxto porque le esté causando efectos adversos graves.
Cómo tomar Venclyxto
- Tome los comprimidos junto con una comida aproximadamente a la misma hora cada día
- Trague los comprimidos enteros con un vaso de agua
- No debe masticar, triturar ni partir los comprimidos
- Durante los primeros días o semanas de tratamiento cuando se aumenta la dosis, debe tomar los comprimidos por la mañana para facilitar el seguimiento mediante analíticas de sangre, si fuera necesario.
Si vomita después de tomar Venclyxto, no tome una dosis adicional ese día. Tome la siguiente dosis a la hora habitual al día siguiente. Si tiene problemas para tomar este medicamento, informe a su médico.
Beba mucha agua
Si tiene LLC
Es muy importante que beba mucha agua mientras esté tomando Venclyxto durante las primeras 5 semanas de tratamiento. Esto ayudará a eliminar de la sangre los productos de desecho de las células cancerosas a través de la orina.
Debe empezar a beber como mínimo, de 1,5 a 2 litros de agua al día, dos días antes de comenzar con Venclyxto. Puede además incluir bebidas sin cafeína y sin alcohol en esta cantidad, pero no beba zumos de pomelo, naranja amarga ni carambola (fruta estrella). Debe seguir bebiendo como mínimo de 1,5 a 2 litros de agua el día que empiece a tomar Venclyxto. Beba la misma cantidad de agua (como mínimo de 1,5 a 2 litros al día) dos días antes de que le aumenten la dosis y ese mismo día.
Si su médico considera que usted está en riesgo de sufrir un SLT, es posible que reciba el tratamiento en el hospital para poder administrarle líquidos intravenosos adicionales, si fuera necesario, hacerle análisis de sangre con más frecuencia y revisar si hay efectos adversos. Esta precaución se toma para saber si puede seguir tomando este medicamento de manera segura.
Si tiene LMA
Es muy importante que beba mucha agua mientras esté tomando Venclyxto, especialmente cuando empiece el tratamiento y cuando aumente la dosis. Beber agua ayudará a eliminar de la sangre los productos de desecho de las células cancerosas a través de la orina. Si está en el hospital, su médico o enfermero le administrarán líquidos en vena si es necesario para asegurarse de que esto ocurre.
Si toma más Venclyxto del que debe
Si toma más Venclyxto del que debe, consulte a su médico, farmacéutico o enfermero o acuda al hospital inmediatamente. Lleve consigo los comprimidos y este prospecto.
Si olvidó tomar Venclyxto
- Si han transcurrido menos de 8 horas desde el momento en que habitualmente toma su dosis, tómela lo antes posible.
- Si han transcurrido más de 8 horas desde el momento en que habitualmente toma su dosis, no la tome ese día. Vuelva a su horario normal de toma de la dosis al día siguiente.
- No tome una dosis doble para compensar las dosis olvidadas.
- En caso de duda, consulte a su médico, farmacéutico o enfermero.
Si interrumpe el tratamiento con Venclyxto
No interrumpa el tratamiento con este medicamento a menos que se lo indique su médico. Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran. Este medicamento puede provocar los siguientes efectos adversos graves:
Síndrome de lisis tumoral(frecuente -puede afectar hasta 1 de cada 10 personas)
Deje de tomar Venclyxto y solicite atención médica inmediatamente si nota alguno de los síntomas de SLT:
- fiebre o escalofríos
- náuseas o vómitos
- confusión
- dificultad para respirar
- ritmo cardíaco irregular
- orina oscura o turbia
- sensación inusual de cansancio
- dolor de músculos o molestias en las articulaciones
- crisis epiléptica o convulsiones
- dolor y distensión abdominal
Descenso del número de glóbulos blancos (neutropenia) e infecciones(muy frecuente -puede afectar a más de 1 de cada 10 personas)
Su médico comprobará la cantidad de células en sangre durante el tratamiento con Venclyxto. El descenso de glóbulos blancos puede aumentar el riesgo de infecciones. Los signos pueden ser fiebre, escalofríos, debilidad o confusión, tos, dolor o sensación de quemazón al orinar. Algunas infecciones como neumonía o infección sanguínea (sepsis) pueden ser graves y provocar la muerte. Informe a su médico inmediatamente si tiene signos de infección durante el tratamiento con este medicamento.
Informe a su médico si nota alguno de los siguientes efectos adversos:
Si tiene LLC
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas)
- neumonía
- infección de las vías respiratorias altas (los signos son secreción nasal, dolor de garganta o tos)
- diarrea
- náuseas o vómitos
- estreñimiento
- cansancio
También se puede observar lo siguiente en el análisis de sangre:
- descenso del número de glóbulos rojos
- descenso del número de glóbulos blancos llamados linfocitos
- aumento de la cantidad de potasio
- aumento de una sal del organismo electrolito (un electrolito) llamado fosfato
- disminución de la cantidad de calcio
Frecuentes(pueden afectar hasta 1 de cada 10 personas)
- infección grave en la sangre (sepsis)
- infección urinaria
- descenso del número de glóbulos blancos con fiebre (neutropenia febril)
También se puede observar lo siguiente en el análisis de sangre:
- aumento de la cantidad de creatinina
- aumento de la cantidad de urea
Si tiene LMA
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas)
- sensación de mareo (náuseas o vómitos)
- diarrea
- llagas en la boca
- sensación de cansancio o debilidad
- infección del pulmón o la sangre
- pérdida de apetito
- dolor en las articulaciones
- mareo o desfallecimiento
- dolor de cabeza
- dificultad para respirar
- sangrado
- presión arterial baja
- infección del tracto urinario
- pérdida de peso
- dolo en la barriga (dolor abdominal)
Los análisis de sangre podrían mostrar:
- un número más bajo de plaquetas (trombocitopenia)
- un número más bajo de glóbulos blancos con fiebre (neutropenia febril)
- un número más bajo de glóbulos rojos (anemia)
- un nivel más alto de bilirrubina total
- un nivel bajo de potasio en sangre
Frecuentes(pueden afectar hasta 1 de cada 10 personas)
- cálculos biliares o infección de la vesícula biliar
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Venclyxto
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta del blister y el envase, después de CAD.:
No requiere condiciones especiales de conservación.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Venclyxto
El principio activo es venetoclax.
- Venclyxto 10 mg comprimidos recubiertos con película: Cada comprimido recubierto con película contiene 10 mg de venetoclax.
- Venclyxto 50 mg comprimidos recubiertos con película: Cada comprimido recubierto con película contiene 50 mg de venetoclax.
- Venclyxto 100 mg comprimidos recubiertos con película: Cada comprimido recubierto con película contiene 100 mg de venetoclax.
Los demás componentes son:
- En el núcleo del comprimido: copovidona (K 28), polisorbato 80 (E433), sílice coloidal anhidra (E551), hidrogenofosfato de calcio anhidro (E341 (ii)), estearil fumarato de sodio.
En la cubierta pelicular:
- Venclyxto 10 mg comprimidos recubiertos con película: óxido de hierro amarillo (E172), alcohol polivinílico (E1203), dióxido de titanio (E171), macrogol 3350 (E1521), talco (E553b).
- Venclyxto 50 mg comprimidos recubiertos con película: óxido de hierro amarillo (E172), óxido de hierro rojo (E172), óxido de hierro negro (E172), alcohol polivinílico (E1203), dióxido de titanio (E171), macrogol 3350 (E1521), talco (E553b).
- Venclyxto 100 mg comprimidos recubiertos con película: óxido de hierro amarillo (E172), alcohol polivinílico (E1203), dióxido de titanio (E171), macrogol 3350 (E1521), talco (E553b).
Aspecto del producto y contenido del envase
Venclyxto 10 mg comprimido recubierto con película es de color amarillo pálido, redondo, de 6 mm de diámetro, marcado con una V en una cara y 10 en la otra.
Venclyxto 50 mg comprimido recubierto con película es de color beige, oblongo, de 14 mm de largo, marcado con una V en una cara y 50 en la otra.
Venclyxto 100 mg comprimido recubierto con película es de color amarillo pálido, oblongo, de 17,2 mm de largo, marcado con una V en una cara y 100 en la otra.
Los comprimidos de Venclyxto se presentan en blísters o frascos contenidos en estuches del siguiente modo:
Venclyxto 10 mg comprimidos recubiertos con película:
- 10 comprimidos (5 blísters cada uno con 2 comprimidos)
- 14 comprimidos (7 blísters cada uno con 2 comprimdos)
Venclyxto 50 mg comprimidos recubiertos con película:
- 5 comprimidos (5 blísters cada uno con 1 comprimido)
- 7 comprimidos (7 blísters cada uno con 1 comprimdo)
Venclyxto 100 mg comprimidos recubiertos con película:
- 7 comprimidos (7 blísters cada uno con 1 comprimido)
- 14 comprimidos (7 blísters cada uno con 2 comprimdos)
- 112 (4 x 28) comprimidos (4 envases de 7 blísters cada uno con 4 comprimidos)
- 360 comprimidos (3 frascos de 120 comprimidos cada uno)
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización yresponsable de la fabricación
AbbVie Deutschland GmbH & Co. KG
Knollstrasse
67061 Ludwigshafen
Alemania
AbbVie S.r.l.
S.R. 148 Pontina, km 52 SNC
04011 Campoverde di Aprilia (Latina)
Italia
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien AbbVie SA Tél/Tel: +32 10 477811 | Lietuva AbbVie UAB Tel: +370 5 205 3023 |
| Luxembourg/Luxemburg AbbVie SA Belgique/Belgien Tél/Tel: +32 10 477811 |
Ceská republika AbbVie s.r.o. Tel: +420 233 098 111 | Magyarország AbbVie Kft. Tel:+36 1 455 8600 |
Danmark AbbVie A/S Tlf: +45 72 30‑20‑28 | Malta V.J.Salomone Pharma Limited Tel: +356 22983201 |
Deutschland AbbVie Deutschland GmbH & Co. KG Tel: 00800 222843 33 (gebührenfrei) Tel: +49 (0) 611 / 1720‑0 | Nederland AbbVie B.V. Tel: +31 (0)88 322 2843 |
Eesti AbbVie OÜ Tel: +372 623 1011 | Norge AbbVie AS Tlf: +47 67 81 80 00 |
Ελλ?δα AbbVie ΦΑΡΜΑΚΕΥΤΙΚΗ Α.Ε. Τηλ: +30 214 4165 555 | Österreich AbbVie GmbH Tel: +43 1 20589‑0 |
España AbbVie Spain, S.L.U. Tel: +34 91 384 09 10 | Polska AbbVie Sp. z o.o. Tel.: +48 22 372 78 00 |
France AbbVie Tél: +33 (0) 1 45 60 13 00 | Portugal AbbVie, Lda. Tel: +351 (0)21 1908400 |
Hrvatska AbbVie d.o.o. Tel: + 385 (0)1 5625 501 | România AbbVie S.R.L. Tel: +40 21 529 30 35 |
Ireland AbbVie Limited Tel: +353 (0)1 4287900 | Slovenija AbbVie Biofarmacevtska družba d.o.o. Tel: +386 (1)32 08 060 |
Ísland Vistor hf. Tel: +354 535 7000 | Slovenská republika AbbVie s.r.o. Tel: +421 2 5050 0777 |
Italia AbbVie S.r.l. Tel: +39 06 928921 | Suomi/Finland AbbVie Oy Puh/Tel: +358 (0)10 2411 200 |
Κ?προς Lifepharma (Z.A.M.) Ltd Τηλ: +357 22 34 74 40 | Sverige AbbVie AB Tel: +46 (0)8 684 44 600 |
Latvija AbbVie SIA Tel: +371 67605000 |
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos http://www.ema.europa.eu.
En la página web de la Agencia Europea de Medicamentos puede encontrarse este prospecto en todas las lenguas de la Unión Europea/Espacio Económico Europeo.
Para escuchar este prospecto o solicitar una copia en braille, en tamaño de fuente grande o en audio, diríjase al representante local del titular de la autorización de comercialización.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a VENCLYXTO 10 MG COMPRIMIDOS RECUBIERTOS CON PELICULAForma farmacéutica: COMPRIMIDO, 100 mgPrincipio activo: VenetoclaxFabricante: Abbvie Deutschland Gmbh & Co. KgRequiere recetaForma farmacéutica: COMPRIMIDO, 50 mgPrincipio activo: VenetoclaxFabricante: Abbvie Deutschland Gmbh & Co. KgRequiere recetaForma farmacéutica: CAPSULA, 0.5 mgPrincipio activo: AnagrelidaFabricante: Aurovitas Spain, S.A.U.Requiere receta
Médicos online para VENCLYXTO 10 MG COMPRIMIDOS RECUBIERTOS CON PELICULA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de VENCLYXTO 10 MG COMPRIMIDOS RECUBIERTOS CON PELICULA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes