
TEBARAT 1 mg/ml NASAL SPRAY SOLUTION

How to use TEBARAT 1 mg/ml NASAL SPRAY SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Tebarat 1 mg/ml nasal spray solution
azelastine hydrochloride
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What is Tebarat and what is it used for
- What you need to know before you use Tebarat
- How to use Tebarat
- Possible side effects
- Storage of Tebarat
- Contents of the pack and further information
1. What is Tebarat and what is it used for
Tebarat contains azelastine hydrochloride which belongs to a group of medicines called antihistamines.
Antihistamines prevent the effects of substances such as histamine and other substances that the body produces as part of an allergic reaction, which cause symptoms such as sneezing, runny nose, itching or nasal congestion. Azelastine hydrochloride also has an additional anti-inflammatory effect.
Tebarat is used for the treatment of symptoms of seasonal allergic rhinitis and acute exacerbations (attacks) of perennial allergic rhinitis in adults, adolescents and children from 6 years of age.
2. What you need to know before you use Tebarat
Do not use Tebarat
If you are allergic to the active substance (azelastine hydrochloride) or to any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist before starting to use Tebarat if you are not sure if your disorders are caused by an allergy.
Children and adolescents
Tebarat is not recommended for children under 6 years of age.
Other medicines and Tebarat
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines.
No specific interactions have been studied.
Pregnancy, breastfeeding and fertility
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Due to the nasal route of administration and the low doses administered, minimal systemic exposure can be expected. However, as with all medicines, precautions should be taken during use in pregnancy and breastfeeding.
Driving and using machines
No effects on the ability to drive or use machines have been described for Tebarat.
3. How to use Tebarat
Follow exactly the administration instructions of this medicine given by your doctor or pharmacist. If you are in doubt, consult your doctor or pharmacist again.
The recommended dose (adults and children over 6 years) is one spray (0.14 ml) of Tebarat into each nostril, twice a day. This corresponds to a daily dose of 0.56 mg of azelastine hydrochloride.
Use in elderly people: no specific studies have been conducted.
Instructions for use


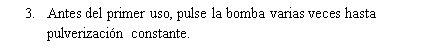
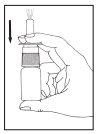

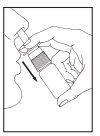
If you use more Tebarat than you should
If you have sprayed too much Tebarat into your nostrils, consult your doctor or pharmacist.
With the nasal route of administration, no overdose reactions are expected.
Animal studies show that toxic doses can produce symptoms on the Central Nervous System, such as excitement, tremors, convulsions. If this occurs in humans, symptomatic and supportive treatment will be initiated. If the overdose is recent, gastric lavage is recommended.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount ingested.
If you forget to use Tebarat
Do not take a double dose to make up for forgotten doses.
If you forget to use your medicine, use it as soon as you remember and put the next dose 12 hours later, if necessary.
If you stop using Tebarat
Do not stop treatment abruptly.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
These effects include:
- Frequent (may affect up to 1 in 10 people): after administration, a bitter taste may appear due to incorrect application, for example, with the head too tilted back during administration.
- Uncommon (may affect up to 1 in 100 people): nasal mucosa irritation with symptoms such as itching, sneezing, epistaxis (small nasal bleeding) may occur.
- Rare (may affect up to 1 in 1,000 people): nausea.
- Very rare (may affect up to 1 in 10,000 people): hypersensitivity, dizziness, fatigue, weakness, rash, pruritus, urticaria.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if it is possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Medicines Monitoring System http://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Tebarat
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and carton after EXP. The expiry date is the last day of the month stated.
Do not use Tebarat after 60 days of opening the bottle.
This medicine does not require any special storage conditions.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and further information
Composition of Tebarat
- The active substance is azelastine hydrochloride 1 mg per ml.
- The other ingredients are hypromellose, disodium edetate, citric acid, sodium hydrogen phosphate heptahydrate, sodium chloride and purified water.
Appearance and packaging
Tebarat is a colorless and transparent solution presented in 20 ml amber glass bottles with a dosing valve, containing a nasal spray solution.
Marketing authorisation holder
Laboratorios Salvat, S.A.
Gall, 30-36 - 08950
Esplugues de Llobregat
Barcelona – Spain
Manufacturer
Pharmaloop, S.L.
C/ Bolivia, 15 – Polig Industrial Azque
28806 Alcalá de Henares
Madrid – Spain
Laboratorios Salvat, S.A.
C/ Gall, 30-36
08950 – Esplugues de Llobregat (Barcelona)
Spain
This medicinal product is authorised in the Member States of the European Economic Area under the following names:
Spain Tebarat 1 mg/ml nasal spray solution
Italy Tebarat
Portugal Tebarat 1 mg/ml solução para pulverização nasal
Date of last revision of this leaflet:
August 2022
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
- Country of registration
- Average pharmacy price11.33 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TEBARAT 1 mg/ml NASAL SPRAY SOLUTIONDosage form: NASAL PRODUCT, 0.1 g azelastine hydrochloride/100 mlActive substance: azelastineManufacturer: Cooper Consumer Health B.V.Prescription requiredDosage form: NASAL PRODUCT, 1.5 mg/mlActive substance: azelastineManufacturer: Cooper Consumer Health B.V.Prescription requiredDosage form: NASAL PRODUCT, 1.5 mg/mlActive substance: azelastineManufacturer: Cooper Consumer Health B.V.Prescription required
Online doctors for TEBARAT 1 mg/ml NASAL SPRAY SOLUTION
Discuss questions about TEBARAT 1 mg/ml NASAL SPRAY SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











