
ROPIVACAINE B.BRAUN 7.5 mg/ml INJECTABLE SOLUTION

How to use ROPIVACAINE B.BRAUN 7.5 mg/ml INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
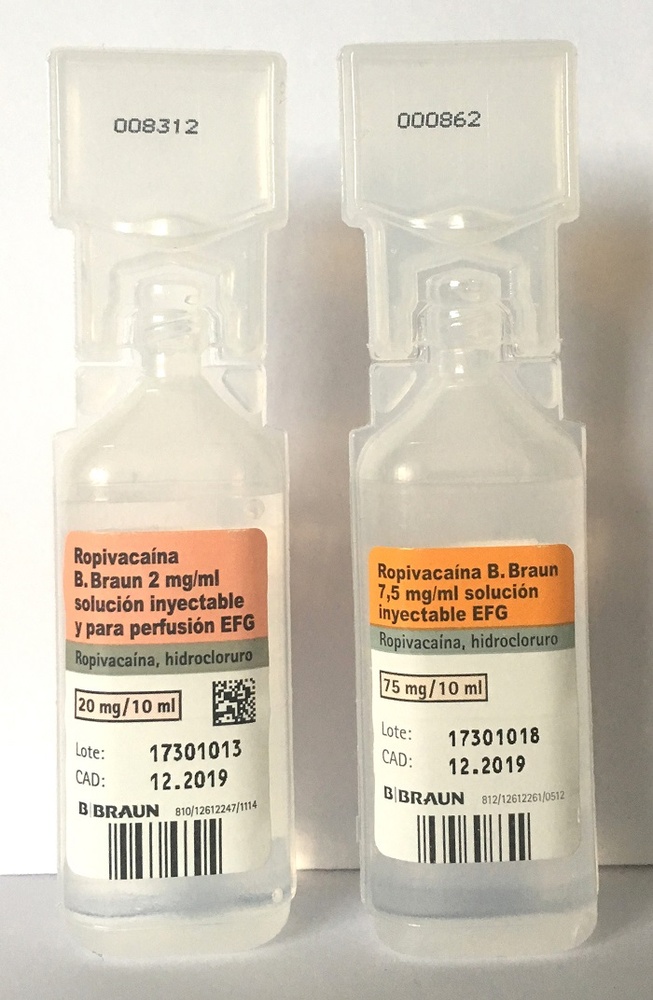
Ropivacaína B. Braun 7.5 mg/ml Solution for Injection EFG
Ropivacaína, Hydrochloride
Read all of this leaflet carefully before you start receiving this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Ropivacaína B. Braun and what is it used for
- What you need to know before you receive Ropivacaína B. Braun
- How Ropivacaína B. Braun will be administered
- Possible side effects
- Storage of Ropivacaína B. Braun
- Contents of the pack and further information
1. What is Ropivacaína B. Braun and what is it used for
The active substance is Ropivacaína Hydrochloride.
Ropivacaína B. Braun belongs to a group of medicines called local anesthetics (medicines to numb). Ropivacaína B. Braun solution for injection is used
- in adults and adolescents (over 12 years) to numb (anesthetize) parts of their body. It is used to prevent pain or relieve pain. It can be used to
- numb parts of the body during surgery, including childbirth by cesarean section.
- relieve pain during childbirth, after surgery, or after an accident.
2. What you need to know before you receive Ropivacaína B. Braun
Ropivacaína B. Braun should not be administered to you
- Ifyou are allergicto Ropivacaína Hydrochlorideor to any of the other ingredientsof this medicine (listed in section 6). An allergic reaction may include rash, itching, difficulty breathing, or swelling of the face, lips, throat, or tongue.
- If you are allergic to any other local anesthetic of the same class (e.g., lidocaine or bupivacaine).
- For injection into a blood vessel to numb a specific area of your body, or into the neck of the uterus to relieve pain during childbirth.
- If you have been told you have a decrease in blood volume (hypovolemia).
If you are not sure if any of the above applies to you, talk to your doctor before you receive Ropivacaína B. Braun.
Warnings and precautions
Tell your doctor before you start receiving Ropivacaína B. Braun, inform your doctor:
- If you have heart, liver, or kidney problems. Your doctor may need to adjust the dose of Ropivacaína B. Braun.
- If you have ever been told you have a rare blood pigment disease called "porphyria" or if someone in your family has it. Your doctor may need to administer a different anesthetic medicine.
- If you have a weak state of health due to advanced age or other reasons.
- About any illness or medical problem you have or have had in the past.
Children
In children up to 12 years, other concentrations (2 mg/ml, 5 mg/ml) may be more suitable.
Using Ropivacaína B. Braun with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
This is especially important with the following medicines, which may make the effects of Ropivacaína B. Braun stronger:
- other local anesthetics (e.g., lidocaine),
- strong painkillers (e.g., morphine),
- medicines used to treat irregular heartbeat (e.g., amiodarone, mexiletine).
Prolonged use of ropivacaína should be avoided if you are being given:
- medicines used to treat depression (e.g., fluvoxamine),
- antibiotics used to treat bacterial infections (e.g., enoxacin).
It may be suitable for you to receive Ropivacaína B. Braun while you are on these treatments. Your doctor needs to know to be able to decide what is appropriate for you.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before you receive this medicine. It is not known if ropivacaína can pass into breast milk or if it can harm the baby.
Driving and using machines
Ropivacaína B. Braun may cause drowsiness and affect your ability to react. After receiving this medicine, do not drive, use machines, or work in hazardous situations until the next day.
Ropivacaína B. Braun contains sodium
This medicine contains 2.9 mg of sodium (main component of cooking/table salt) per ml. This is equivalent to 0.15% of the maximum recommended daily sodium intake for an adult.
3. How to use Ropivacaína B. Braun
This medicine will be administered to you by an expert doctor or under their supervision.
Ropivacaína B. Braun will be administered to you as an injection. The part of your body where it will be applied will depend on the reason why you are receiving this medicine.
Your doctor will administer this medicine to you in one of the following places:
- The part of your body that needs to be numbed.
- Near the part of your body that needs to be numbed.
- In a distant area from the part of your body that needs to be numbed. This is the case if you receive an epidural injection or infusion in the middle or lower back near the spine.
While you are receiving Ropivacaína B. Braun, you will be closely monitored by healthcare professionals. This medicine makes the nerves stop transmitting pain messages to the brain. You will stop feeling pain, heat, or cold in the area where it is used, but you may still feel other sensations like pressure or touch.
Dosage
Your doctor will decide the dose of Ropivacaína B. Braun that will be administered to you. The dosage depends on the type of pain relief you need and other factors such as your build, age, and physical condition.
If you are administered more Ropivacaína B. Braun than you should
Since this medicine will be administered to you by a doctor under carefully controlled conditions, it is unlikely that you will be administered a higher dose or miss a dose.
Serious side effects from receiving too much Ropivacaína B. Braun require special treatment. Your doctor is specialized to act in these situations.
The first signs of having received too much Ropivacaína B. Braun are usually the following:
- You feel dizzy or lightheaded.
- Numbness of the lips and around the mouth.
- Numbness of the tongue.
- Hearing problems.
- Vision problems (sight).
Your doctor will stop administering this medicine as soon as these signs appear to reduce the risk of serious side effects. This means that if you experience any of them or think you have received too much Ropivacaína B. Braun, you must inform your doctor immediately.
More serious side effects from receiving too much of this medicine include speech problems, muscle spasms, tremors, convulsions, seizures, and loss of consciousness.
In case of acute toxicity, healthcare professionals will take immediate corrective actions.
If you have any further questions about the use of this medicine, ask your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
All medicines, including Ropivacaína B. Braun, can very rarely produce allergic reactionssuch as anaphylaxis, including anaphylactic shock (may affect up to 1 in 1,000 people). You must inform your doctor immediatelyif you experience any of the following symptoms after receiving this medicine:
- sudden appearance of rash, itching, or hives;
- swelling of the eyelids, face, lips, tongue, throat, or other parts of the body;
- difficulty breathing, sudden shortness of breath;
- a feeling of loss of consciousness.
Other possible side effects
Very common (may affect more than 1 in 10 people)
- Low blood pressure (hypotension) (you may feel dizzy or lightheaded).
- Feeling sick (nausea).
Common (may affect up to 1 in 10 people)
- Tingling.
- Dizziness.
- Feeling sick (vomiting).
- Slow or fast heart rate (bradycardia, tachycardia).
- High blood pressure (hypertension).
- High body temperature (fever) or chills (shivering).
- Back pain.
- Headache.
- Difficulty urinating.
Uncommon (may affect up to 1 in 100 people)
- Anxiety.
- Fainting.
- Breathing difficulties.
- Low body temperature (hypothermia).
- Some symptoms may occur if the injection is accidentally administered into a blood vessel or if too much Ropivacaína B. Braun is administered (see also section "If you are administered more Ropivacaína B. Braun than you should" above). These include seizures, feeling dizzy or lightheaded, numbness of the lips and around the mouth, numbness of the tongue, hearing problems, vision problems, speech problems, stiff muscles, decreased sensitivity or sensation in the skin, and tremors.
Rare (may affect up to 1 in 1,000 people)
- Heart attack (cardiac arrest).
- Irregular heartbeat (arrhythmia).
Frequency not known (cannot be estimated from the available data)
- Sudden movements (dyskinesia).
Possible side effects observed with other local anesthetics that may also be produced by Ropivacaína B. Braun:
- Nerve damage. This can rarely cause permanent problems.
- The whole body may become numb (anesthetized) if too much Ropivacaína B. Braun is injected into the spinal fluid.
Children
In infants and children, the side effects are the same as in adults, except for low blood pressure, which occurs less frequently in infants and children (affects up to 1 in 10 children) and feeling sick, which occurs more frequently in children (affects more than 1 in 10 children).
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. You can also report side effects directly to the Spanish Medicines and Healthcare Products Agency (AEMPS) at http://www.aemps.gob.es/. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Ropivacaína B. Braun
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and label after "EXP". The expiry date refers to the last day of the month shown.
Do not freeze.
Your doctor or pharmacist is responsible for storing this medicine. They are also responsible for the proper disposal of unused medicine.
6. Contents of the pack and further information
Composition of Ropivacaína B. Braun
The active substance is Ropivacaína Hydrochloride.
1 ml of Ropivacaína B. Braun contains 7.5 mg of Ropivacaína Hydrochloride (as Ropivacaína Hydrochloride monohydrate).
1 ampoule of 10 ml of solution for injection contains 75 mg of Ropivacaína Hydrochloride (as Ropivacaína Hydrochloride monohydrate).
1 ampoule of 20 ml of solution for injection contains 150 mg of Ropivacaína Hydrochloride (as Ropivacaína Hydrochloride monohydrate).
The other ingredients are sodium chloride, hydrochloric acid 0.36% (for pH adjustment), sodium hydroxide 0.4% (for pH adjustment), and water for injections.
Appearance and pack contents
Ropivacaína B. Braun is a clear and colorless solution for injection.
- Ropivacaína B. Braun 7.5 mg/ml solution for injection is available in:
- Polyethylene ampoules of 10 ml in packs of 20.
- Polyethylene ampoules of 20 ml in packs of 20.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
- Braun Melsungen AG
Carl-Braun-Strasse 1 Postal address:
34212 Melsungen, Germany 34209 Melsungen, Germany
Phone: +49/5661/71-0
Fax: +49/5661/71-4567
This medicine is authorized in the Member States of the European Economic Area under the following names:
Austria, Germany, Luxembourg: Ropivacain-HCl B. Braun 7.5 mg/ml Injektionslösung
Bulgaria: Ropivacain HCI B. Braun 7.5 mg/ml solution for injection
Denmark: Ropivacain B. Braun 7.5 mg/ml
Estonia: Ropivacaine B. Braun 7.5 mg/ml
- Finland: Ropivacaine B. Braun 7.5 mg/ml injektioneste, liuos
- France: Ropivacaine B Braun 7.5 mg/ml, solution injectable en ampoule
- Greece: Ropivacain HCI B. Braun 7.5 mg/ml solution for injection
- Italy: Ropivacaina B. Braun 7.5 mg/ml soluzione iniettabile
- Latvia: Ropivacaine B. Braun 7.5 mg/ml šķīdums injekcijām
- Lithuania: Ropivacaine B. Braun 7.5 mg/ml injekcinis tirpalas
- Netherlands: Ropivacaïne HCl B. Braun 7.5 mg/ml, oplossing voor injectie
- Norway: Ropivacain B. Braun 7.5 mg/ml injeksjonsvæske, opplosning
- Portugal: Ropivacaína B. Braun 7.5 mg/ml solução injetável
- Spain: Ropivacaina B. Braun 7.5 mg/ml solución inyectable
- Sweden: Ropivacaine B. Braun 7.5 mg/ml injektionsvätska, lösning
Date of last revision of this leaflet:10/2023.
Detailed and up-to-date information on this medicine is available on the website of the Spanish Medicines and Healthcare Products Agency (AEMPS) http://www.aemps.gob.es/
------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
Method of administration
Careful aspiration should be performed before and during injection to prevent intravascular injection. When a higher dose is to be administered, a test dose of lidocaine with adrenaline (epinephrine) is recommended. Accidental intravascular injection can be recognized by a temporary increase in heart rate and accidental intrathecal injection by signs of spinal block.
Ropivacaína Hydrochloride should be injected slowly or in increasing doses, at a rate of 25-50 mg/min, while constantly monitoring the patient's vital functions and maintaining verbal contact with them. If symptoms of toxicity appear, administration should be stopped immediately.
Warnings
Regional anesthesia procedures should always be performed in an area equipped with adequate facilities and staffed by specialized personnel. Equipment and medicines necessary for monitoring and emergency resuscitation should be available at all times.
Patients who are to undergo a major block should be in optimal general health and have an intravenous line available before the block procedure is performed.
The doctor in charge should take the necessary precautions to avoid intravascular injection (see section 4.2 of the Summary of Product Characteristics) and be adequately trained and familiar with the diagnosis and treatment of side effects, toxicity, and other complications (see sections 4.8 and 4.9 of the Summary of Product Characteristics) such as accidental subarachnoid injection, which can cause high spinal block with apnea and hypotension. Seizures have occurred more frequently after brachial plexus block and epidural block. This is probably due to accidental intravascular injection or rapid absorption from the injection site.
Blockade of peripheral nerve trunks may involve the administration of a large volume of local anesthetic in highly vascularized areas, often close to large vessels, where there is a greater risk of intravascular injection and/or rapid systemic absorption, which can lead to high plasma concentrations.
Patients with hypovolemia due to any cause may suddenly develop severe hypotension during epidural anesthesia, regardless of the local anesthetic used.
Handling
Disposal of unused medicine and all materials that have come into contact with it should be carried out in accordance with local regulations.
For single use only.
The medicine should be inspected visually before use.
It should only be used if the solution is clear and colorless and if the ampoules and their closures are intact.
Shelf life after first opening of the container
From a microbiological point of view, unless the opening method excludes the risk of microbial contamination, the product should be used immediately.
If not used immediately, the in-use storage times and conditions are the responsibility of the user.
See the Summary of Product Characteristics for instructions on incompatibilities and all information related to prescribing.
.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ROPIVACAINE B.BRAUN 7.5 mg/ml INJECTABLE SOLUTIONDosage form: INJECTABLE, 100 mgActive substance: ropivacaineManufacturer: Altan Pharmaceuticals SaPrescription requiredDosage form: INJECTABLE PERFUSION, 2 mg/mlActive substance: ropivacaineManufacturer: Altan Pharmaceuticals SaPrescription requiredDosage form: INJECTABLE, 75 mgActive substance: ropivacaineManufacturer: Altan Pharmaceuticals SaPrescription required
Online doctors for ROPIVACAINE B.BRAUN 7.5 mg/ml INJECTABLE SOLUTION
Discuss questions about ROPIVACAINE B.BRAUN 7.5 mg/ml INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















