Инструкция по применению РИНВОК 45 мг ТАБЛЕТКИ ПРОЛОНГИРОВАННОГО ВЫСВОБОЖДЕНИЯ
Введение
Проспект: информация для пациента
РΙΝВОК 15 мг пролонгированные таблетки
РΙΝВОК 30мг пролонгированные таблетки
РΙΝВОК 45мг пролонгированные таблетки
упадацитиниб
Прочитайте весь проспект внимательно перед началом приема этого лекарства, поскольку он содержит важную информацию для вас.
- Сохраните этот проспект, поскольку вам может потребоваться прочитать его снова.
- Если у вас есть какие-либо вопросы, проконсультируйтесь с вашим врачом, фармацевтом или медсестрой.
- Это лекарство было назначено только вам, и вы не должны давать его другим людям, даже если они имеют те же симптомы, что и вы, поскольку оно может нанести им вред.
- Если вы испытываете побочные эффекты, проконсультируйтесь с вашим врачом, фармацевтом или медсестрой, даже если это побочные эффекты, которые не указаны в этом проспекте. См. раздел 4.
Содержание проспекта
- Что такое РΙΝВОК и для чего он используется
- Что вам нужно знать перед началом приема РΙΝВОК
- Как принимать Р-INFRINGEMENT
- Возможные побочные эффекты
- Хранение Р-INFRINGEMENT
- Содержание упаковки и дополнительная информация
1. Что такое РΙΝВОК и для чего он используется
Р-INFRINGEMENT содержит активное вещество упадацитиниб. Он принадлежит к группе лекарств, называемых ингибиторами Янус-киназы. Уменьшая активность фермента в организме, называемого "Янус-киназой", Р-INFRINGEMENT уменьшает воспаление следующих заболеваний:
- Ревматоидный артрит
- Псориатический артрит
- Аксиальный спондилоартрит
- Нерадиографический аксиальный спондилоартрит
- Анкилозирующий спондилит (АС, радиографический аксиальный спондилоартрит)
- Гигантоклеточный артериит
- Атопический дерматит
- Язвенный колит
- Болезнь Крона
Ревматоидный артрит
Р-INFRINGEMENT используется для лечения взрослых с ревматоидным артритом. Ревматоидный артрит - это заболевание, которое вызывает воспаление суставов. Если у вас ревматоидный артрит средней или тяжелой степени, вам могут назначить другие лекарства, одно из которых обычно является метотрексатом. Если эти лекарства не работают достаточно хорошо, вам будет назначен Р-INFRINGEMENT в одиночку или в комбинации с метотрексатом для лечения ревматоидного артрита.
Р-INFRINGEMENT может помочь уменьшить боль, скованность и воспаление суставов, уменьшить усталость и замедлить прогрессию повреждения костей и хряща суставов. Эти эффекты могут облегчить ваши повседневные занятия и улучшить качество жизни.
Псориатический артрит
Р-INFRINGEMENT используется для лечения взрослых с псориатическим артритом. Псориатический артрит - это заболевание, которое вызывает воспаление суставов и псориаз. Если у вас активный псориатический артрит, вам могут назначить другие лекарства. Если эти лекарства не работают достаточно хорошо, вам будет назначен Р-INFRINGEMENT в одиночку или в комбинации с метотрексатом для лечения псориатического артрита.
Р-INFRINGEMENT может помочь уменьшить боль, скованность и воспаление в и вокруг суставов, боль и скованность позвоночника, кожную сыпь, вызванную псориазом, и усталость, а также может замедлить повреждение костей и хряща суставов. Эти эффекты могут облегчить ваши повседневные занятия и улучшить качество жизни.
Аксиальный спондилоартрит (аксиальный спондилоартрит и анкилозирующий спондилит)
Р-INFRINGEMENT используется для лечения взрослых с аксиальным спондилоартритом. Аксиальный спондилоартрит - это заболевание, которое в основном вызывает воспаление позвоночника. Если у вас активный аксиальный спондилоартрит, вам могут назначить другие лекарства. Если эти лекарства не работают достаточно хорошо, вам будет назначен Р-INFRINGEMENT для лечения аксиального спондилоартрита.
Р-INFRINGEMENT может помочь уменьшить боль в нижней части спины, скованность и воспаление позвоночника. Эти эффекты могут облегчить ваши повседневные занятия и улучшить качество жизни.
Гигантоклеточный артериит
Р-INFRINGEMENT используется для лечения взрослых с гигантоклеточным артериитом. Гигантоклеточный артериит - это заболевание, которое вызывает воспаление кровеносных сосудов, обычно поражающее средние и крупные артерии в голове, шее и руках.
Р-INFRINGEMENT может помочь контролировать симптомы и признаки гигантоклеточного артериита, включая головную боль, чувствительность в коже головы, боль в челюсти и усталость. Эти эффекты могут облегчить ваши повседневные занятия и улучшить качество жизни. Гигантоклеточный артериит обычно лечится лекарствами, называемыми стероидами. Они обычно эффективны, но могут иметь побочные эффекты, если принимаются в высоких дозах или в течение длительного времени. Уменьшение дозы стероидов также может привести к обострению гигантоклеточного артериита. Добавление Р-INFRINGEMENT к лечению означает, что стероиды можно использовать в течение более короткого периода, и, таким образом, контролировать гигантоклеточный артериит.
Атопический дерматит
Р-INFRINGEMENT используется для лечения взрослых и подростков от 12 лет с атопическим дерматитом средней или тяжелой степени, также известным как атопический экзема. Р-INFRINGEMENT можно использовать с лекарствами для экземы, наносимыми на кожу, или использовать в одиночку.
Прием Р-INFRINGEMENT может улучшить состояние кожи и уменьшить зуд и обострения. Р-INFRINGEMENT может помочь улучшить симптомы боли, тревоги и депрессии, которые могут иметь люди с атопическим дерматитом. Р-INFRINGEMENT может помочь улучшить нарушения сна и общее качество жизни.
Язвенный колит
Язвенный колит - это воспалительное заболевание толстой кишки. Р-INFRINGEMENT используется для лечения взрослых с язвенным колитом, которые не ответили на предыдущее лечение или не перенесли его.
Р-INFRINGEMENT может помочь уменьшить симптомы и признаки заболевания, включая кровавый стул, боль в животе и частоту посещения туалета. Эти эффекты могут облегчить ваши повседневные занятия и уменьшить усталость.
Болезнь Крона
Болезнь Крона - это воспалительное заболевание, которое может поражать любую часть пищеварительного тракта, но чаще всего поражает кишечник. Р-INFRINGEMENT используется для лечения взрослых с болезнью Крона, которые не ответили на предыдущее лечение или не перенесли его.
Р-INFRINGEMENT может помочь уменьшить симптомы и признаки заболевания, включая частоту посещения туалета, боль в животе и воспаление слизистой оболочки кишечника. Эти эффекты могут облегчить ваши повседневные занятия и уменьшить усталость.
2. Что вам нужно знать перед началом приема Р-INFRINGEMENT
Не принимайте Р-INFRINGEMENT
- если вы аллергичны к упадацитинибу или любому другому компоненту этого лекарства (указанному в разделе 6)
- если у вас есть тяжелая инфекция (например, пневмония или инфекция кожи, вызванная бактериями)
- если у вас есть активная туберкулез (ТБ)
- если у вас есть тяжелые проблемы с печенью
- если вы беременны (см. раздел Беременность, лактация и контрацепция).
Предостережения и меры предосторожности
Проконсультируйтесь с вашим врачом или фармацевтом перед и во время лечения Р-INFRINGEMENT, если:
- у вас есть инфекция или вы часто болеете. Сообщите вашему врачу, если у вас есть симптомы, такие как температура, раны, повышенная усталость или проблемы с зубами, поскольку они могут быть признаками инфекции. Р-INFRINGEMENT может уменьшить способность вашего организма бороться с инфекциями и может ухудшить существующую инфекцию или увеличить вероятность того, что вы заразитесь новой инфекцией. Если у вас диабет или вам 65 лет или старше, вы можете иметь более высокий риск заражения инфекциями
- у вас была туберкулез или вы были в тесном контакте с кем-то, у кого туберкулез. Ваш врач проведет тест на туберкулез перед началом приема Р-INFRINGEMENT и может повторить тест во время лечения
- у вас была инфекция, вызванная вирусом герпеса (герпес зостер), поскольку Р-INFRINGEMENT может вызвать его повторное появление. Сообщите вашему врачу, если у вас появилась болезненная кожная сыпь с пузырьками, поскольку это может быть признаком герпеса зостер
- у вас была гепатит Б или С
- вы были недавно вакцинированы или планируете вакцинацию (иммунизацию) - это связано с тем, что не рекомендуется введение живых вакцин во время приема Р-INFRINGEMENT
- у вас есть или были рак, вы курите или курили в прошлом, поскольку ваш врач оценит с вами, подходит ли Р-INFRINGEMENT для вас
- у пациентов, принимающих Р-INFRINGEMENT, наблюдался рак кожи, не связанный с меланомой. Ваш врач может рекомендовать вам регулярные осмотры кожи во время приема Р-INFRINGEMENT. Сообщите вашему врачу, если появляются новые кожные поражения во время или после лечения или если изменяется внешний вид существующих поражений
- у вас есть или были проблемы с сердцем, поскольку ваш врач оценит с вами, подходит ли Р-INFRINGEMENT для вас
- у вас есть проблемы с печенью
- у вас были ранее тромбы в венах ног (глубокая венозная тромбоз) или легких (пульмональная эмболия) или у вас повышенный риск их развития (например, если у вас была недавно проведена крупная операция, вы принимаете гормональные контрацептивы/заместительную гормональную терапию, если у вас или ваших близких родственников выявлена какая-либо аномалия свертываемости крови). Ваш врач оценит с вами, подходит ли Р-INFRINGEMENT для вас. Сообщите вашему врачу, если у вас появляются внезапные симптомы, такие как одышка или трудности с дыханием, боль в груди или верхней части спины, отек в ноге или руке, боль или чувствительность при прикосновении к ноге или изменение цвета кожи в ноге или руке, поскольку это может быть признаком тромбов в венах
- у вас внезапно появляются нарушения зрения. Проконсультируйтесь с врачом немедленно, если у вас появляются внезапные симптомы, такие как размытое зрение или частичная или полная потеря зрения, поскольку это может быть признаком нарушения кровотока в глазах
- у вас есть проблемы с почками
- у вас есть боль в животе неизвестной причины, у вас была дивертикулит (воспаление небольших мешочков в слизистой оболочке кишечника) или язвы в желудке или кишечнике, или вы принимаете нестероидные противовоспалительные препараты
- вы регулярно наблюдаете таблетку или части таблетки в кале.
Если вы заметите любой из следующих серьезных побочных эффектов, сообщите вашему врачу немедленно:
- симптомы, такие как сыпь (крапивница), трудности с дыханием или отек губ, языка или горла, могут указывать на аллергию. Некоторые пациенты, принимающие Р-INFRINGEMENT, испытывали серьезные аллергические реакции. Если во время приема Р-INFRINGEMENT у вас появляются какие-либо из этих симптомов, прекратите прием Р-INFRINGEMENT и немедленно обратитесь за медицинской помощью
- сильная боль в животе, особенно сопровождаемая температурой, тошнотой и рвотой.
Анализы крови
Вам потребуется сдать анализ крови перед началом приема Р-INFRINGEMENT или во время приема. Это делается для проверки наличия у вас низкого уровня красных кровяных телец (анемия), низкого уровня белых кровяных телец (нейтропения или лимфопения), высокого уровня жира в крови (холестерина) или высокого уровня печеночных ферментов. Анализы проводятся для проверки того, что лечение Р-INFRINGEMENT не вызывает проблем.
Пациенты пожилого возраста
Существует более высокий риск инфекций у пациентов 65 лет и старше. Сообщите вашему врачу немедленно, если вы заметите любой признак или симптом инфекции.
Пациенты 65 лет и старше могут иметь более высокий риск инфекций, проблем с сердцем, включая инфаркт, и некоторых видов рака. Ваш врач оценит с вами, подходит ли Р-INFRINGEMENT для вас.
Дети и подростки
Не рекомендуется использовать Р-INFRINGEMENT у детей с атопическим дерматитом моложе 12 лет или подростков с весом менее 30 кг. Это не было изучено у этих пациентов.
Не рекомендуется использовать Р-INFRINGEMENT у детей и подростков моложе 18 лет с ревматоидным артритом, псориатическим артритом, аксиальным спондилоартритом (аксиальным спондилоартритом и анкилозирующим спондилитом), язвенным колитом или болезнью Крона. Это не было изучено у этой возрастной группы.
Другие лекарства и Р-INFRINGEMENT
Сообщите вашему врачу или фармацевту, если вы принимаете, недавно принимали или можете принять любое другое лекарство. Некоторые лекарства могут уменьшить эффективность Р-INFRINGEMENT или увеличить риск побочных эффектов. Очень важно, чтобы вы сообщили вашему врачу или фармацевту, если вы принимаете любое из следующих:
- лекарства для лечения грибковых инфекций (например, итраконазол, позаконазол или вориконазол)
- лекарства для лечения бактериальных инфекций (например, кларитромицин)
- лекарства для лечения синдрома Кушинга (например, кетоконазол)
- лекарства для лечения туберкулеза (например, рифампицин)
- лекарства для лечения судорог или эпилептических приступов (например, фенитоин)
- лекарства, влияющие на вашу иммунную систему (например, азатиоприн, 6-меркаптопурин, циклоспорин и такролимус)
- лекарства, которые могут увеличить риск перфорации желудочно-кишечного тракта или дивертикулита, такие как нестероидные противовоспалительные препараты (обычно используемые для лечения болезненных и/или воспалительных заболеваний мышц или суставов), и/или опиоиды (используемые для лечения сильной боли), и/или кортикостероиды (обычно используемые для лечения воспалительных заболеваний)
- лекарства для лечения диабета или если у вас диабет. Ваш врач может решить, нужно ли вам меньше лекарства для лечения диабета во время приема упадацитиниба.
Если у вас есть любой из этих случаев или вы не уверены, проконсультируйтесь с вашим врачом или фармацевтом перед приемом Р-INFRINGEMENT.
Беременность, лактация и фертильность
Беременность
Р-INFRINGEMENT не должен использоваться во время беременности.
Лактация
Если вы кормите грудью или планируете кормить грудью, проконсультируйтесь с вашим врачом перед приемом этого лекарства. Вы не должны использовать Р-INFRINGEMENT во время кормления грудью, поскольку неизвестно, проходит ли это лекарство в грудное молоко. Вы и ваш врач должны решить, будете ли вы кормить грудью или принимать Р-INFRINGEMENT. Вы не должны делать и то, и другое.
Фертильность
Если вы женщина детородного возраста, вы должны использовать эффективный метод контрацепции, чтобы избежать беременности во время приема Р-INFRINGEMENT и в течение как минимум 4 недель после последней дозы Р-INFRINGEMENT. Если вы забеременеете в это время, вы должны сообщить об этом вашему врачу немедленно.
Вы должны сообщить врачу, если ваша дочь имеет первую менструацию во время приема Р-INFRINGEMENT.
Вождение и использование машин
Не驾驶айте транспортные средства и не используйте машины, если вы чувствуете головокружение или вращение (вертиго) при приеме Р-INFRINGEMENT, пока эти симптомы не пройдут.
3. Как принимать РИНВОК
Следуйте точно инструкциям по применению этого лекарства, указанным вашим врачом или фармацевтом.В случае сомнений проконсультируйтесь снова с вашим врачом или фармацевтом.
Количество, которое необходимо принимать
Если у вас ревматоидный артрит, псориатический артрит, аксиальный спондилоартрит (аксиальный спондилоартрит не радиографический и анкилозирующий спондилит) или гигантоклеточный артериит
Рекомендуемая доза составляет одну таблетку по 15 мг один раз в день.
Если у вас атопический дерматит
Взрослые (от 18 до 64 лет):
Рекомендуемая доза составляет 15 мг или 30 мг, назначаемая вашим врачом, в виде одной таблетки один раз в день.
Ваш врач может увеличить или уменьшить вашу дозу в зависимости от того, как вы реагируете на лечение.
Подростки (от 12до 17лет) с весом не менее 30кг:
Рекомендуемая доза составляет одну таблетку по 15 мг один раз в день. Ваш врач может увеличить вашу дозу до одной таблетки по 30 мг один раз в день, в зависимости от того, как вы реагируете на лечение.
Пациенты пожилого возраста:
Если вам 65 лет или более, рекомендуемая доза составляет 15 мг один раз в день.
Если у вас язвенный колит
Рекомендуемая доза составляет одну таблетку по 45 мг один раз в день в течение 8 недель. Ваш врач решит, продлить ли начальную дозу 45 мг в течение 8 недель (всего 16 недель), за которой следует одна таблетка по 15 мг или 30 мг один раз в день для долгосрочного лечения. Ваш врач может увеличить или уменьшить вашу дозу в зависимости от того, как вы реагируете на лекарство.
Пациенты пожилого возраста:
Если вам 65 лет или более, рекомендуемая доза составляет 15 мг один раз в день для долгосрочного лечения.
Ваш врач может уменьшить вашу дозу, если у вас есть нарушения функции почек или если вам назначены другие лекарства.
Если у вас болезнь Крона
Рекомендуемая доза составляет одну таблетку по 45 мг один раз в день в течение 12 недель. Это будет за которым следует одна таблетка по 15 мг или 30 мг один раз в день для долгосрочного лечения. Ваш врач может увеличить или уменьшить вашу дозу в зависимости от того, как вы реагируете на лекарство.
Пациенты пожилого возраста:
Если вам 65 лет или более, рекомендуемая доза составляет 15 мг один раз в день для долгосрочного лечения.
Ваш врач может уменьшить вашу дозу, если у вас есть нарушения функции почек или если вам назначены другие лекарства.
Как принимать лекарство
- Проглотите таблетку целиком с водой. Не разламывайте, не дробите, не жуйте и не разрывайте таблетку перед проглатыванием, поскольку это может изменить количество лекарства, поступающего в ваш организм.
- Чтобы помочь вам запомнить, что необходимо принимать РИНВОК, принимайте его в одно и то же время каждый день.
- Таблетки можно принимать с пищей или без нее.
- Не принимайте десикант.
- Избегайте продуктов или напитков, содержащих грейпфрут, пока вы принимаете (или проходите лечение с) РИНВОК, поскольку они могут сделать побочные эффекты более вероятными, увеличивая количество лекарства в вашем организме.
Если вы приняли слишком много РИНВОК
Если вы приняли слишком много РИНВОК, проконсультируйтесь с вашим врачом. Вы можете испытать некоторые из побочных эффектов, описанных в разделе 4.
Если вы забыли принять РИНВОК
- Если вы забыли принять одну дозу, примите ее как можно скорее.
- Если вы забыли принять свою дозу в течение целого дня, просто пропустите пропущенную дозу и примите одну дозу на следующий день, как обычно.
- Не принимайте двойную дозу, чтобы компенсировать пропущенные дозы.
Если вы прекратите лечение с РИНВОК
Не прекращайте принимать РИНВОК, если только ваш врач не скажет вам, что необходимо прекратить его прием.
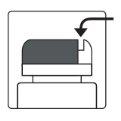
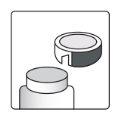
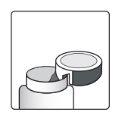
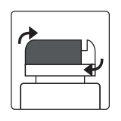
Как открыть флакон
| Устройство для резки алюминия- на крышке флакона |
|
1а.Снимите крышку флакона, нажав вниз и, не отпуская, поверните крышку против часовой стрелки. 1б.Переверните крышку и поместите устройство для резки рядом с краем алюминиевого уплотнения. |
|
|
|
|
Если у вас есть какие-либо другие вопросы о использовании этого лекарства, проконсультируйтесь с вашим врачом или фармацевтом.
4. Возможные побочные эффекты
Как и все лекарства, это лекарство может вызывать побочные эффекты, хотя не все люди испытывают их.
Серьезные побочные эффекты
Сообщите вашему врачу или обратитесь за медицинской помощью немедленно, если вы испытываете любой из следующих признаков:
- инфекция, такая как опоясывающий герпес или болезненная сыпь с пузырьками (опоясывающий герпес) - частые (могут повлиять на до 1 из 10 человек).
- легочная инфекция (пневмония), которая может вызвать затруднение дыхания, лихорадку и кашель с мокротой - частые (могут повлиять на до 1 из 10 человек).
- инфекция крови (сепсис) - редкие (могут повлиять на до 1 из 100 человек).
- аллергическая реакция (давление в груди, свист, отек губ, языка или горла, крапивница) - редкие (могут повлиять на до 1 из 100 человек).
Другие побочные эффекты
Сообщите вашему врачу, если вы испытываете любой из следующих побочных эффектов:
Очень частые(могут повлиять на более 1 из 10 человек)
- инфекции горла и носа
- акне
Частые(могут повлиять на до 1 из 10 человек)
- рак кожи не-меланома
- кашель
- лихорадка
- жар (простой герпес)
- чувство дискомфорта в желудке (тошнота)
- повышение уровня фермента, называемого креатинкиназой, наблюдаемого в анализах крови
- низкое количество белых кровяных клеток в анализах крови
- высокие уровни холестерина (тип жира в крови), наблюдаемые в анализах
- высокие уровни печеночных ферментов, наблюдаемые в анализах крови (признак проблем с печенью)
- прибавка в весе
- воспаление (отек) волосяных фолликулов
- грипп (инфлюэнца)
- анемия
- боль в животе (абдомен)
- усталость (необычное чувство усталости и слабости)
- головная боль (головная боль была очень частой при гигантоклеточном артериите)
- крапивница
- инфекция мочевыводящих путей
- сыпь
- чувство вращения (вертиго)
- головокружение
- инфекция легких (бронхит)
- отек ног и рук (периферический отек)
Редкие(могут повлиять на до 1 из 100 человек)
- оральная кандидоз (белые пятна во рту)
- повышенные уровни триглицеридов (тип жира) в крови, наблюдаемые в анализах
- дивертикулит (болезненное воспаление небольших мешочков в слизистой оболочке кишечника)
- гastrointestinalная перфорация (отверстие в кишечнике)
Дополнительные побочные эффекты у подростков с атопическим дерматитом
Частые:
- бородавки (папиллома кожи)
Сообщение о побочных эффектах
Если вы испытываете любой побочный эффект, проконсультируйтесь с вашим врачом, фармацевтом или медсестрой, даже если это возможные побочные эффекты, которые не перечислены в этом листке-вкладыше. Вы также можете сообщить о них напрямую через национальную систему уведомления, включенную в Приложение V. Сообщая о побочных эффектах, вы можете внести свой вклад в предоставление более полной информации о безопасности этого лекарства.
5. Хранение РИНВОК
Храните это лекарство в недоступном для детей месте.
Не используйте это лекарство после даты истечения срока годности, указанной на блистере и на коробке после "EXP".
Это лекарство не требует специальной температуры хранения.
Храните в оригинальном блистере или флаконе и с плотно закрытой крышкой, чтобы защитить его от влаги.
Лекарства не должны выбрасываться в канализацию или мусор. Спросите вашего фармацевта, как утилизировать упаковку и лекарства, которые вам больше не нужны. Таким образом, вы поможете защитить окружающую среду.
6. Содержание упаковки и дополнительная информация
Состав РИНВОК
Активное вещество - упадацитиниб.
РИНВОК 15 мг пролонгированные таблетки
- Каждая пролонгированная таблетка содержит упадацитиниб гемигидрат, эквивалентный 15 мг упадацитинибу.
- Другие компоненты являются:
- Ядро таблетки: микрокристаллическая целлюлоза, маннитол, яблочная кислота, гипромеллоза, коллоидный алюминиевый силикат, стеарат магния.
- Покрытие: поливиниловый спирт, макрогол, тальк, диоксид титана (E171), оксид железа красный (E172), оксид железа черный (E172).
РИНВОК 30 мг пролонгированные таблетки
- Каждая пролонгированная таблетка содержит упадацитиниб гемигидрат, эквивалентный 30 мг упадацитинибу.
- Другие компоненты являются:
- Ядро таблетки: микрокристаллическая целлюлоза, маннитол, яблочная кислота, гипромеллоза, коллоидный алюминиевый силикат, стеарат магния.
- Покрытие: поливиниловый спирт, макрогол, тальк, диоксид титана (E171), оксид железа красный (E172).
РИНВОК 45 мг пролонгированные таблетки
- Каждая пролонгированная таблетка содержит упадацитиниб гемигидрат, эквивалентный 45 мг упадацитинибу.
- Другие компоненты являются:
- Ядро таблетки: микрокристаллическая целлюлоза, маннитол, яблочная кислота, гипромеллоза, коллоидный алюминиевый силикат, стеарат магния.
- Покрытие: поливиниловый спирт, макрогол, тальк, диоксид титана (E171), оксид железа желтый (E172) и оксид железа красный (E172).
Внешний вид продукта и содержание упаковки
РИНВОК 15 мг пролонгированные таблетки
РИНВОК 15 мг пролонгированные таблетки - это фиолетовые, овальные и двояковыпуклые таблетки, гравированные "a15" на одной стороне.
Таблетки поставляются в блистерах или флаконах.
РИНВОК доступен в упаковках по 28 или 98 пролонгированных таблеток и в многодозных упаковках по 84 пролонгированные таблетки, каждая из которых содержит 3 упаковки по 28 пролонгированных таблеток.
Каждый календарный блистер содержит 7 таблеток.
РИНВОК доступен в флаконах с десикантом, содержащих 30 пролонгированных таблеток, каждая упаковка содержит 1 флакон (упаковка по 30 таблеток) или 3 флакона (упаковка по 90 таблеток).
РИНВОК 30 мг пролонгированные таблетки
РИНВОК 30 мг пролонгированные таблетки - это красные, овальные и двояковыпуклые таблетки, гравированные "a30" на одной стороне.
Таблетки поставляются в блистерах или флаконах.
РИНВОК доступен в упаковках по 28 или 98 пролонгированных таблеток.
Каждый календарный блистер содержит 7 таблеток.
РИНВОК доступен в флаконах с десикантом, содержащих 30 пролонгированных таблеток, каждая упаковка содержит 1 флакон (упаковка по 30 таблеток) или 3 флакона (упаковка по 90 таблеток).
РИНВОК 45 мг пролонгированные таблетки
РИНВОК 45 мг пролонгированные таблетки - это желтые или желто-пятнистые, овальные и двояковыпуклые таблетки, гравированные "a45" на одной стороне.
Таблетки поставляются в блистерах или флаконах.
РИНВОК доступен в упаковках по 28 пролонгированных таблеток.
Каждый календарный блистер содержит 7 таблеток.
РИНВОК доступен в флаконах с десикантом, содержащих 28 пролонгированных таблеток, каждая упаковка содержит 1 флакон.
Возможно, не все размеры упаковок будут продаваться.
Владелец разрешения на маркетинг
AbbVie Deutschland GmbH & Co. KG
Knollstrasse
67061 Людвигсхафен
Германия
Производитель
AbbVie S.r.l.
S.R. 148 Pontina, km 52 SNC
04011 Камповерде ди Априлия (Латина)
Италия
AbbVie Logistics B.V.
Зёйдерзеелан 53
Зволле, 8017 JV,
Нидерланды
Вы можете запросить больше информации о этом лекарстве, обратившись к местному представителю владельца разрешения на маркетинг:
Бельгия/Белгия/Бельгия AbbVie SA Тел: +32 10 477811 | Литва AbbVie UAB Тел: +370 5 205 3023 | |
| Люксембург/Люксембург AbbVie SA Бельгия/Бельгия Тел: +32 10 477811 | |
Чехия AbbVie s.r.o. Тел: +420 233 098 111 | Венгрия AbbVie Kft. Тел.: +36 1 455 8600 | |
Дания AbbVie A/S Тел: +45 72 30-20-28 | Мальта V.J.Salomone Pharma Limited Тел: +356 2122017 | |
Германия AbbVie Deutschland GmbH & Co. KG Тел: 00800 222843 33 (бесплатно) Тел: +49 (0) 611 / 1720-0 | Нидерланды AbbVie B.V. Тел: +31 (0)88 322 2843 | |
Эстония AbbVie OU Тел: +372 623 1011 | Норвегия AbbVie AS Тел: +47 67 81 80 00 | |
Греция AbbVie ΦΑΡΜΑΚΕΥΤΙΚΗ Α.Ε. Тел: +30 214 4165 555 | Австрия AbbVie GmbH Тел: +43 1 20589-0 | |
Испания AbbVie Spain, S.L.U. Тел.: +34 91 384 09 10 | Польша AbbVie Polska Sp. z o.o. Тел.: +48 22 372 78 00 | |
Франция AbbVie Тел: +33 (0) 1 45 60 13 00 | Португалия AbbVie, Lda. Тел: +351 (0)21 1908400 | |
Хорватия AbbVie d.o.o. Тел + 385 (0)1 5625 501 | Румыния AbbVie S.R.L. Тел: +40 21 529 30 35 | |
Ирландия AbbVie Limited Тел: +353 (0)1 4287900 | Словения AbbVie Biofarmacevtska družba d.o.o. Тел: +386 (1)32 08 060 | |
Исландия Vistor Тел: +354 535 7000 | Словакия AbbVie s.r.o. Тел: +421 2 5050 0777 | |
Италия AbbVie S.r.l. Тел: +39 06 928921 | Финляндия AbbVie Oy Тел: +358 (0)10 2411 200 | |
Кипр Lifepharma (Z.A.M.) Ltd Тел.: +357 22 34 74 40 | Швеция AbbVie AB Тел: +46 (0)8 684 44 600 | |
Латвия AbbVie SIA Тел: +371 67605000 |
Дата последнего пересмотра этого листка-вкладыша:
Другие источники информации
Подробная информация о этом лекарстве доступна на сайте Европейского агентства по лекарствам: http://www.ema.europa.eu.
Вы также можете просмотреть подробную и актуальную информацию о этом продукте, отсканировав QR-код, представленный ниже, или на внешней упаковке с помощью смартфона. Та же информация также доступна по следующему URL: www.rinvoq.eu.
Включить QR-код
Чтобы прослушать или запросить копию этого листка-вкладыша в <Брайле>, <большом размере шрифта> или прослушать его в <аудио>, свяжитесь с местным представителем владельца разрешения на маркетинг.
- Страна регистрации
- Активное вещество
- Требуется рецептДа
- Производитель
- Информация носит справочный характер и не является медицинской рекомендацией. Перед приемом любых препаратов проконсультируйтесь с врачом. Oladoctor не несет ответственности за медицинские решения, принятые на основе этого контента.
- Аналоги РИНВОК 45 мг ТАБЛЕТКИ ПРОЛОНГИРОВАННОГО ВЫСВОБОЖДЕНИЯФорма выпуска: ТАБЛЕТКА С МОДИФИЦИРОВАННЫМ ВЫСВОБОЖДЕНИЕМ, 15 мгАктивное вещество: upadacitinibПроизводитель: Abbvie Deutschland Gmbh & Co. KgТребуется рецептФорма выпуска: ТАБЛЕТКА С МОДИФИЦИРОВАННЫМ ВЫСВОБОЖДЕНИЕМ, 30 мгАктивное вещество: upadacitinibПроизводитель: Abbvie Deutschland Gmbh & Co. KgТребуется рецептФорма выпуска: ТАБЛЕТКА, 100 мгАктивное вещество: filgotinibПроизводитель: Alfasigma S.P.A.Требуется рецепт
Врачи онлайн по РИНВОК 45 мг ТАБЛЕТКИ ПРОЛОНГИРОВАННОГО ВЫСВОБОЖДЕНИЯ
Консультация по дозировке, побочным эффектам, взаимодействиям, противопоказаниям и продлению рецепта на РИНВОК 45 мг ТАБЛЕТКИ ПРОЛОНГИРОВАННОГО ВЫСВОБОЖДЕНИЯ – по решению врача и с учетом местных правил.