
PRIVENAX 110 mg HARD CAPSULES

How to use PRIVENAX 110 mg HARD CAPSULES
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Privenax 110 mg Hard Capsules EFG
dabigatran etexilate
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Privenax and what is it used for
- What you need to know before you take Privenax
- How to take Privenax
- Possible side effects
- Storage of Privenax
- Contents of the pack and other information
1. What is Privenax and what is it used for
This medicine contains the active substance dabigatran etexilate and belongs to a group of medicines called anticoagulants. It works by blocking a substance in the body involved in the formation of blood clots.
Dabigatran is used in adults to:
- prevent the formation of blood clots in the veins after knee or hip replacement surgery.
- prevent the formation of blood clots in the brain (stroke) and in other blood vessels of the body if you have a type of irregular heartbeat called non-valvular atrial fibrillation and have at least one additional risk factor.
- treat blood clots in the veins of your legs and lungs and to prevent them from happening again in the veins of your legs and lungs.
Dabigatran is used in children to:
- treat and prevent blood clots.
2. What you need to know before you take Privenax
Do not take Privenax
- if you are allergic to dabigatran etexilate or any of the other ingredients of this medicine (listed in section 6).
- if your kidney function is severely reduced.
- if you are currently bleeding.
- if you have any disease in an organ of the body that increases the risk of severe bleeding (e.g. stomach ulcer, injury or bleeding in the brain, recent brain or eye surgery).
- if you are prone to bleeding. This tendency may be congenital, of unknown cause or caused by other medicines.
- if you are taking medicines to prevent blood clotting (e.g. warfarin, rivaroxaban, apixaban or heparin), except when switching from one anticoagulant treatment to another, while having a venous or arterial catheter and being administered heparin through this catheter to keep it open or while your normal heartbeat is being restored through a procedure called catheter ablation for atrial fibrillation.
- if your liver function is severely reduced or you have a life-threatening liver disease.
- if you are taking oral ketoconazole or itraconazole, medicines used to treat fungal infections.
- if you are taking oral cyclosporin, a medicine used to prevent organ rejection after a transplant.
- if you are taking dronedarone, a medicine used to treat irregular heartbeat.
- if you are taking a combination product of glecaprevir and pibrentasvir, an antiviral medicine used to treat hepatitis C.
- if you have been implanted with an artificial heart valve that requires permanent anticoagulant treatment.
Warnings and precautions
Talk to your doctor before starting to take dabigatran. During treatment with this medicine, you may also need to talk to your doctor if you experience any symptoms or if you need to have surgery.
Tell your doctorif you have or have had any disorder or disease, especially any of the following:
- If you have an increased risk of bleeding, for example:
- if you have recently had bleeding.
- if you have had a tissue removal (biopsy) in the last month.
- if you have had a severe injury (e.g. a bone fracture, a head injury or any injury that required surgical treatment).
- if you have inflammation of the esophagus or stomach.
- if you have problems with gastric juice reflux in the esophagus.
- if you are taking medicines that may increase the risk of bleeding. See "Other medicines and dabigatran" below.
- if you are using anti-inflammatory medicines such as diclofenac, ibuprofen or piroxicam.
- if you have a heart infection (bacterial endocarditis).
- if you know you have reduced kidney function or if you are dehydrated (symptoms include feeling thirsty and passing small amounts of dark-colored urine/concentrated urine with foam).
- if you are over 75 years old.
- if you are an adult patient and weigh 50 kg or less.
- only if used in children: if the child has a brain infection or around the brain.
- If you have had a heart attack or if you have been diagnosed with diseases that increase the risk of having a heart attack.
- If you have a liver disease associated with changes in blood tests. The use of dabigatran is not recommended in this case.
Be careful with dabigatran
- If you need to have surgery:
In this case, dabigatran should be temporarily stopped due to a higher risk of bleeding during and after surgery. It is very important that you take this medicine before and after surgery exactly at the times indicated by your doctor.
- If surgery requires the placement of a catheter or injection into the spinal column (e.g. for epidural or spinal anesthesia or for pain relief):
- It is very important that you take dabigatran before and after surgery exactly at the times indicated by your doctor.
- Tell your doctor immediately if you experience numbness or weakness in your legs or bowel or bladder problems after the end of anesthesia, as this situation requires urgent attention.
- If you fall or injure yourself during treatment, especially if you hit your head. Seek urgent medical attention. You may need a doctor to examine you, as you may have a higher risk of bleeding.
- If you know you have a disease called antiphospholipid syndrome (an immune system disorder that increases the risk of blood clots), tell your doctor so that they can decide if it is necessary to modify the treatment.
Other medicines and dabigatran
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. In particular, you must tell your doctor before taking dabigatran if you are taking any of the following medicines:
- Medicines to reduce blood clotting (e.g. warfarin, phenprocoumon, acenocoumarol, heparin, clopidogrel, prasugrel, ticagrelor, rivaroxaban, acetylsalicylic acid)
- Medicines for the treatment of fungal infections (e.g. ketoconazole, itraconazole), unless they are only applied to the skin
- Medicines used to treat irregular heartbeat (e.g. amiodarone, dronedarone, quinidine, verapamil)
If you are using medicines that contain amiodarone, quinidine or verapamil, your doctor may instruct you to use a reduced dose of dabigatran depending on the disease for which it was prescribed. See section 3.
- Medicines for the prevention of organ rejection after a transplant (e.g. tacrolimus, cyclosporin)
- A combination product of glecaprevir and pibrentasvir (an antiviral medicine used to treat hepatitis C)
- Anti-inflammatory and pain-relieving medicines (e.g. acetylsalicylic acid, ibuprofen, diclofenac)
- St. John's Wort, a herbal medicine for depression
- Antidepressant medicines called selective serotonin reuptake inhibitors or selective serotonin and norepinephrine reuptake inhibitors
- Rifampicin or clarithromycin (two antibiotics)
- Antiviral medicines for HIV (e.g. ritonavir)
- Certain medicines for the treatment of epilepsy (e.g. carbamazepine, phenytoin)
Pregnancy and breast-feeding
The effects of dabigatran on pregnancy and the fetus are not known. You should not use dabigatran if you are pregnant unless your doctor tells you it is safe to do so. If you are of childbearing age, you should avoid becoming pregnant during treatment with this medicine.
Breast-feeding is not recommended during treatment with dabigatran.
Driving and using machines
Dabigatran has no known effects on the ability to drive and use machines.
3. How to take Privenax
Privenax capsules can be used in adults and children aged 8 years or older who are able to swallow the capsules whole.
Follow the instructions for administration of this medicine exactly as indicated by your doctor. If you are unsure, consult your doctor again.
Take this medicine as recommended for the following situations:
Prevention of blood clot formation after knee or hip replacement surgery
The recommended dose is 220 mg once a day(administered in the form of 2 capsules of 110 mg).
If your kidney function is reducedby more than half or if you are 75 years of age or older, the recommended dose is 150 mg once a day(administered in the form of 2 capsules of 75 mg).
If you are using medicines that contain amiodarone, quinidine or verapamil, the recommended dose is 150 mg once a day(administered in the form of 2 capsules of 75 mg).
If you are using medicines that contain verapamil and your kidney function is reducedby more than half, you should be indicated a reduced dose of dabigatran of 75 mgbecause your risk of bleeding may increase.
In both types of surgery, treatment should not be started if there is bleeding at the surgical site. If treatment cannot be started until the day after surgery, dosing should be started with 2 capsules once a day.
After knee replacement surgery
You should start treatment with dabigatran 1-4 hours after surgery, taking a single capsule. After that, you should take 2 capsules once a day for a total of 10 days.
After hip replacement surgery
You should start treatment with dabigatran 1-4 hours after surgery, taking a single capsule. After that, you should take 2 capsules once a day for a total of 28-35 days.
Prevention of stroke or systemic embolism due to blood clot formation in patients with irregular heartbeat and treatment of blood clots in the veins of your legs and lungs, including prevention of recurrence of blood clots in the veins of your legs and lungs
The recommended dose is 300 mg administered in the form of one 150 mg capsule twice a day.
If you are 80 years of age or older, the recommended dose of dabigatran is 220 mg administered in the form of one 110 mg capsule twice a day.
If you are using medicines that contain verapamil, you should be indicated a reduced dose of dabigatran of 220 mg taken in the form of one 110 mg capsule twice a day, because your risk of bleeding may increase.
If you have a potentially higher risk of bleeding, your doctor may decide to prescribe a dose of 220 mg administered in the form of one 110 mg capsule twice a day.
You can continue taking this medicine if you need to restore your normal heartbeat through a procedure called cardioversion. Take dabigatran as indicated by your doctor.
If you have been implanted with a medical device (vascular endoprosthesis) in a blood vessel to keep it open in a procedure called percutaneous coronary intervention with vascular endoprosthesis, you may receive treatment with dabigatran once your doctor has decided that normal blood clotting control has been achieved. Take this medicine as indicated by your doctor.
Treatment of blood clots and prevention of recurrence of blood clots in children
Dabigatran should be taken twice a day, one dose in the morning and one dose in the evening, approximately at the same time every day. The administration interval should be as close as possible to 12 hours.
The recommended dose depends on age and weight. Your doctor will determine the correct dose. Your doctor may adjust the dose during treatment. Continue using all other medicines unless your doctor tells you to stop using any.
Table 1 shows the single and total daily doses of Privenax in milligrams (mg). The doses depend on the weight in kilograms (kg) and age in years of the patient.
Weight/Age Combinations | Single Dose in mg | Total Daily Dose in mg | |
Weight in kg | Age in years | ||
11 to less than 13 kg | 8 to less than 9 years | 75 | 150 |
13 to less than 16 kg | 8 to less than 11 years | 110 | 220 |
16 to less than 21 kg | 8 to less than 14 years | 110 | 220 |
21 to less than 26 kg | 8 to less than 16 years | 150 | 300 |
26 to less than 31 kg | 8 to less than 18 years | 150 | 300 |
31 to less than 41 kg | 8 to less than 18 years | 185 | 370 |
41 to less than 51 kg | 8 to less than 18 years | 220 | 440 |
51 to less than 61 kg | 8 to less than 18 years | 260 | 520 |
61 to less than 71 kg | 8 to less than 18 years | 300 | 600 |
71 to less than 81 kg | 8 to less than 18 years | 300 | 600 |
81 kg or more | 10 to less than 18 years | 300 | 600 |
Doses that require combinations of more than one capsule:
300 mg: two 150 mg capsules or
four 75 mg capsules
260 mg: one 110 mg capsule plus one 150 mg capsule or
one 110 mg capsule plus two 75 mg capsules
220 mg: two 110 mg capsules
185 mg: one 75 mg capsule plus one 110 mg capsule
150 mg: one 150 mg capsule or
two 75 mg capsules
How to take Privenax
Privenax can be taken with or without food. The capsule should be swallowed whole with a glass of water to ensure release in the stomach. Do not break, chew or open the capsule to take only its contents, as this may increase the risk of bleeding.
Instructions for opening the blisters
The following images illustrate how to remove the Privenax capsules from the blister:
1
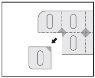 Separate a single blister from the blister strip through the perforated line.
Separate a single blister from the blister strip through the perforated line.
2
 Remove the rear foil and remove the capsule.
Remove the rear foil and remove the capsule.
- Do not press the capsules through the blister foil.
- Do not remove the blister foil until the capsule is needed.
Changing anticoagulant treatment
Do not change your anticoagulant treatment without specific instructions from your doctor.
If you take more Privenax than you should
Taking too much Privenax increases the risk of bleeding. Contact your doctor immediately if you have taken too many capsules of this medication. There are specific treatment options available.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medication and the amount ingested.
If you forget to take Privenax
Prevention of blood clot formation after knee or hip replacement surgery
Take the remaining daily doses of dabigatran at the same time the next day.
Do not take a double dose to make up for missed doses.
Adult use: Prevention of cerebral or systemic vascular obstruction by blood clot formation developed after abnormal heart rhythm and treatment of blood clots in the veins of the legs and lungs, including prevention of recurrence of blood clots in the veins of the legs and lungs
Use in children: Treatment of blood clots and prevention of recurrence of blood clots
A missed dose can be taken up to 6 hours before the next dose.
A missed dose should be omitted if the time remaining before the next dose is less than 6 hours. Do not take a double dose to make up for missed doses.
If you interrupt treatment with Privenax
Take Privenax exactly as prescribed. Do not interrupt your treatment with Privenax without consulting your doctor first, as the risk of developing a blood clot may be higher if treatment is interrupted too soon. Contact your doctor if you experience indigestion after taking this medication.
If you have any other questions about the use of this medication, ask your doctor or pharmacist.
4. Possible adverse effects
Like all medications, this medication can cause adverse effects, although not all people experience them.
Dabigatran acts on blood coagulation; therefore, most adverse effects are related to signs such as bruising or bleeding. Episodes of major or severe bleeding may occur, which are the most serious adverse effects and can cause disability, be potentially life-threatening, or even cause death. In some cases, these bleedings may not be apparent.
If you experience any episode of bleeding that does not stop on its own or if you experience signs of excessive bleeding (exceptional weakness, fatigue, paleness, dizziness, headache, or unexplained swelling), consult your doctor immediately. Your doctor may decide to keep you under close observation or change your medication.
Inform your doctor immediately if you experience a severe allergic reaction that causes difficulty breathing or dizziness.
Possible adverse effects are listed below, grouped according to their frequency.
Prevention of blood clot formation after knee or hip replacement surgery
Frequent (may affect up to 1 in 10 people):
- Decrease in the amount of hemoglobin in the blood (the substance present in red blood cells)
- Anomalies in liver function tests
Uncommon (may affect up to 1 in 100 people):
- Bleeding may occur through the nose, in the stomach or intestine, from the penis/vagina or urinary tract (including blood in the urine that stains the urine pink or red), or under the skin
- Formation of hematomas or bruising after surgery
- Detection of blood in stool in a laboratory test
- Decrease in the number of red blood cells in the blood
- Decrease in the proportion of blood cells
- Allergic reaction
- Vomiting
- Frequent loose or liquid stools
- Feeling of nausea
- Pus discharge from a wound (discharge of liquid from a surgical wound)
- Increased liver enzymes
- Yellowish discoloration of the skin or whites of the eyes, caused by liver or blood problems
Rare (may affect up to 1 in 1,000 people):
- Bleeding
- Bleeding may occur in the brain, at the site of a surgical incision, at the site of an injection, or at the site of a catheter in a vein
- Bloody pus discharge from the site of a catheter in a vein
- Coughing up blood or sputum with blood stains
- Decrease in the number of platelets in the blood
- Decrease in the number of red blood cells in the blood after surgery
- Severe allergic reaction that causes difficulty breathing or dizziness
- Severe allergic reaction that causes swelling of the face or throat
- Skin rash with dark red, prominent, and itchy bumps, caused by an allergic reaction
- Sudden change in skin color and appearance
- Itching
- Ulcer in the stomach or intestine (including ulcer in the esophagus)
- Inflammation of the esophagus and stomach
- Reflux of stomach juice into the esophagus
- Abdominal pain or stomach pain
- Indigestion
- Difficulty swallowing
- Fluid discharge from a wound
- Fluid discharge from a wound after surgery
Frequency not known (frequency cannot be estimated from available data):
- Difficulty breathing or wheezing
- Decrease in the number or even absence of white blood cells (which help fight infections)
- Hair loss
Prevention of cerebral or systemic vascular obstruction by blood clot formation developed after abnormal heart rhythm
Frequent (may affect up to 1 in 10 people):
- Bleeding may occur through the nose, in the stomach or intestine, from the penis/vagina or urinary tract (including blood in the urine that stains the urine pink or red), or under the skin
- Decrease in the number of red blood cells in the blood
- Abdominal pain or stomach pain
- Indigestion
- Frequent loose or liquid stools
- Feeling of nausea
Uncommon (may affect up to 1 in 100 people):
- Bleeding
- Bleeding may occur from hemorrhoids, from the rectum, or in the brain
- Formation of hematomas
- Coughing up blood or sputum with blood stains
- Decrease in the number of platelets in the blood
- Decrease in the amount of hemoglobin in the blood (the substance present in red blood cells)
- Allergic reaction
- Sudden change in skin color and appearance
- Itching
- Ulcer in the stomach or intestine (including ulcer in the esophagus)
- Inflammation of the esophagus and stomach
- Reflux of stomach juice into the esophagus
- Vomiting
- Difficulty swallowing
- Anomalies in liver function tests
Rare (may affect up to 1 in 1,000 people):
- Bleeding may occur in a joint, at the site of a surgical incision, in a wound, at the site of an injection, or at the site of a catheter in a vein
- Severe allergic reaction that causes difficulty breathing or dizziness
- Severe allergic reaction that causes swelling of the face or throat
- Skin rash with dark red, prominent, and itchy bumps, caused by an allergic reaction
- Decrease in the proportion of blood cells
- Increased liver enzymes
- Yellowish discoloration of the skin or whites of the eyes, caused by liver or blood problems
Frequency not known (frequency cannot be estimated from available data):
- Difficulty breathing or wheezing
- Decrease in the number or even absence of white blood cells (which help fight infections)
- Hair loss
Treatment of blood clots in the veins of the legs and lungs, including prevention of recurrence of blood clots in the veins of the legs and/or lungs
Frequent (may affect up to 1 in 10 people):
- Bleeding may occur through the nose, in the stomach or intestine, from the rectum, from the penis/vagina or urinary tract (including blood in the urine that stains the urine pink or red), or under the skin
- Indigestion
Uncommon (may affect up to 1 in 100 people):
- Bleeding
- Bleeding may occur in a joint or in a wound
- Bleeding may occur from hemorrhoids
- Decrease in the number of red blood cells in the blood
- Formation of hematomas
- Coughing up blood or sputum with blood stains
- Allergic reaction
- Sudden change in skin color and appearance
- Itching
- Ulcer in the stomach or intestine (including ulcer in the esophagus)
- Inflammation of the esophagus and stomach
- Reflux of stomach juice into the esophagus
- Feeling of nausea
- Vomiting
- Abdominal pain or stomach pain
- Frequent loose or liquid stools
- Anomalies in liver function tests
- Increased liver enzymes
Rare (may affect up to 1 in 1,000 people):
- Bleeding may occur at the site of a surgical incision, or at the site of an injection, or at the site of a catheter in a vein or from the brain
- Decrease in the number of platelets in the blood
- Severe allergic reaction that causes difficulty breathing or dizziness
- Severe allergic reaction that causes swelling of the face or throat
- Skin rash with dark red, prominent, and itchy bumps, caused by an allergic reaction
- Difficulty swallowing
Frequency not known (frequency cannot be estimated from available data):
- Difficulty breathing or wheezing
- Decrease in the amount of hemoglobin in the blood (the substance present in red blood cells)
- Decrease in the proportion of blood cells
- Decrease in the number or even absence of white blood cells (which help fight infections)
- Yellowish discoloration of the skin or whites of the eyes, caused by liver or blood problems
- Hair loss
In a clinical trial, the rate of heart attacks with dabigatran was numerically higher than with warfarin. The overall incidence was low.
Treatment of blood clots and prevention of recurrence of blood clots in children
Frequent (may affect up to 1 in 10 people):
- Decrease in the number of red blood cells in the blood
- Decrease in the number of platelets in the blood
- Skin rash with dark red, prominent, and itchy bumps, caused by an allergic reaction
- Sudden change in skin color and appearance
- Formation of hematomas
- Nasal bleeding
- Reflux of stomach juice into the esophagus
- Vomiting
- Feeling of nausea
- Frequent loose or liquid stools
- Indigestion
- Hair loss
- Increased liver enzymes
Uncommon (may affect up to 1 in 100 people):
- Decrease in the number of white blood cells (which help fight infections)
- Bleeding may occur in the stomach or intestine, in the brain, in the rectum, from the penis/vagina or urinary tract (including blood in the urine that stains the urine pink or red), or under the skin
- Decrease in the amount of hemoglobin in the blood (the substance present in red blood cells)
- Decrease in the proportion of blood cells
- Itching
- Coughing up blood or sputum with blood stains
- Abdominal pain or stomach pain
- Inflammation of the esophagus and stomach
- Allergic reaction
- Difficulty swallowing
- Yellowish discoloration of the skin or whites of the eyes, caused by liver or blood problems
Frequency not known (frequency cannot be estimated from available data):
- Absence of white blood cells (which help fight infections)
- Severe allergic reaction that causes difficulty breathing or dizziness
- Severe allergic reaction that causes swelling of the face or throat
- Difficulty breathing or wheezing
- Bleeding
- Bleeding may occur in a joint or in a wound, in a surgical incision, at the site of an injection, or at the site of a catheter in a vein
- Bleeding may occur from hemorrhoids
- Ulcer in the stomach or intestine (including ulcer in the esophagus)
- Anomalies in liver function tests
Reporting of adverse effects
If you experience any adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaram.es. By reporting adverse effects, you can help provide more information on the safety of this medication.
5. Storage of Privenax
Keep this medication out of the sight and reach of children.
Do not use this medication after the expiration date stated on the carton or blister pack after "EXP". The expiration date is the last day of the month indicated.
Do not store above 30°C.
Store in the original packaging to protect from moisture.
Medicines should not be disposed of via wastewater or household waste. Place the packaging and any unused medication in the SIGRE collection point at the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medication. This will help protect the environment.
6. Package contents and additional information
Composition of Privenax
- The active ingredient is dabigatran. Each hard capsule contains 110 mg of dabigatran etexilate (as mesilate).
- The other ingredients are tartaric acid, arabic gum, hypromellose 2910, dimeticone 350, talc, and hydroxypropylcellulose
- The capsule shell contains carrageenan, potassium chloride, titanium dioxide (E-171), hypromellose, and blue FD&C 2/indigo carmine (E-132).
Appearance and packaging of the product
Privenax 110 mg are white to pale yellow pellets inside hard blue capsules of size 1.
Privenax is available in packs containing 10 x 1, 30 x 1, or 60 x 1 hard capsules in perforated aluminum/OPA-AL-PVC blister packs.
Not all pack sizes may be marketed.
Marketing authorization holder
Kern Pharma, S.L.
Venus, 72 – Pol. Ind. Colón II
08228 Terrassa - Barcelona
Spain
Manufacturer
Galenicum Health, S.L.U.
Sant Gabriel, 50,
08950 – Esplugues de Llobregat (Barcelona)
Spain
or
SAG Manufacturing S.L.U
Crta. N-I, Km 36
28750 San Agustin de Guadalix,
Madrid – Spain
This medicationis authorized in the Member States of the European Economic Area under the following names:
Malta – Privenax 110 mg hard capsules
Portugal – Dabigatrano etexilato Pharmakern 110 mg capsules
Spain – Privenax 110 mg hard capsules EFG
Date of last revision of this leaflet:June 2024
Updated and detailed information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS): http://www.aemps.gob.es/.
- Country of registration
- Average pharmacy price16.53 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to PRIVENAX 110 mg HARD CAPSULESDosage form: CAPSULE, 110 mgActive substance: dabigatran etexilateManufacturer: Adamed Laboratorios S.L.U.Prescription requiredDosage form: CAPSULE, 150 mgActive substance: dabigatran etexilateManufacturer: Adamed Laboratorios S.L.U.Prescription requiredDosage form: CAPSULE, 75 mgActive substance: dabigatran etexilateManufacturer: Adamed Laboratorios S.L.U.Prescription required
Online doctors for PRIVENAX 110 mg HARD CAPSULES
Discuss questions about PRIVENAX 110 mg HARD CAPSULES, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















