
PHYSIONEAL 35 2.27% GLUCOSE SOLUTION FOR PERITONEAL DIALYSIS

How to use PHYSIONEAL 35 2.27% GLUCOSE SOLUTION FOR PERITONEAL DIALYSIS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
PHYSIONEAL 35 Glucose 1.36% w/v/ 13.6 mg/ml CLEAR-FLEX, Solution for Peritoneal Dialysis
PHYSIONEAL 35 Glucose 2.27% w/v/ 22.7 mg/ml CLEAR-FLEX, Solution for Peritoneal Dialysis
PHYSIONEAL 35 Glucose 3.86% w/v/ 38.6 mg/ml CLEAR-FLEX, Solution for Peritoneal Dialysis
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.Keep this leaflet. You may need to read it again..
- Keep this leaflet, as you may need to read it again.
- If you have any further questions, ask your doctor.
- If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What PHYSIONEAL 35 is and what it is used for
- What you need to know before you use PHYSIONEAL 35
- How to use PHYSIONEAL 35
- Possible side effects
- Storage of PHYSIONEAL 35
- Contents of the pack and other information
1. What PHYSIONEAL 35 is and what it is used for
PHYSIONEAL 35 is a solution for peritoneal dialysis. It removes water and waste products from the blood. It also corrects abnormal levels of various blood components.
PHYSIONEAL 35 contains different concentrations of glucose (1.36%, 2.27%, or 3.86%). The higher the glucose concentration of the solution, the more water will be removed from the blood.
PHYSIONEAL 35 may be prescribed for you if you have:
- temporary or permanent kidney failure
- severe water retention
- severe acid-base disturbances (pH) and electrolyte imbalances in the blood
- certain types of poisoning due to medications for which there are no other available treatments.
The PHYSIONEAL 35 solution has an acidity (pH) similar to that of blood. For this reason, it may be particularly useful if you experience discomfort or pain during the administration of other, more acidic peritoneal dialysis solutions.
2. What you need to know before you use PHYSIONEAL 35
Your doctor should supervise the administration of this product if it is the first time you are using it.
Do not use PHYSIONEAL 35
- if you are allergic to the active substances or to any of the other components of this medicine (listed in section 6).
- if you have any non-correctable surgical problem affecting your abdominal wall or cavity, or an uncorrectable condition that increases the risk of abdominal infections
- if you have a documented loss of peritoneal function due to severe peritoneal scarring.
Treatment with PHYSIONEAL 35 in its CLEAR FLEX packaging is not recommended in some cases:
- children who require fill volumes of 1600 ml
Warnings and precautions
Before using it, you must:
- First, mix the contents of the two chambers by opening the long seal;
- Second, open the short seal (Safety Moon).
- If you infuse the unmixed solution (the long seal between the two chambers has not been opened), you may experience abdominal pain. Drain the solution immediately, use a new mixed bag, and inform your doctor promptly.
- If you do not drain the unmixed solution, the levels of electrolytes or other chemical substances may increase in your blood. This can cause side effects such as confusion, drowsiness, and irregular heartbeat.
Tell your doctor before using PHYSIONEAL 35.
Be particularly careful:
- If you have severe problems affecting the integrity of the abdominal wall or cavity. For example, in the case of a hernia or chronic infection or inflammatory disease affecting the intestines.
- If you have an aortic graft.
- If you have severe breathing difficulties.
- If you experience abdominal pain, elevated body temperature, or notice that the drainage fluid is cloudy or contains particles. This may be a sign of peritonitis (inflamed peritoneum) or infection. You should contact your medical team urgently. Note the batch number of the peritoneal dialysis solution bags you are using and take it, along with the drained fluid bag, to your medical team. Your medical team will decide whether to interrupt treatment or initiate corrective treatment. For example, if you have an infection, your doctor may perform several tests to determine which antibiotic is most suitable for you. Your doctor may give you a broad-spectrum antibiotic that is effective against a wide range of different bacteria until they know what infection you have.
- If you have a high level of lactate in your blood. You are at high risk of lactic acidosis if:
- you have a significant drop in blood pressure
- you have a blood infection
- you have acute kidney failure
- you have a congenital metabolic disorder
- you are taking metformin (a medication used to treat diabetes)
- you are taking medications to treat HIV, especially those called NRTIs.
- If you have diabetes and use this solution, the dose of your medications used to regulate blood sugar levels (e.g., insulin) should be reviewed regularly. In particular, the dose of your diabetes medications should be adjusted when peritoneal dialysis treatment is initiated or changed.
- If you have a corn allergy that may lead to hypersensitivity reactions, including severe allergic reactions known as anaphylaxis. Stop the infusion immediately and drain the solution from the peritoneal cavity.
- You, probably in conjunction with your doctor, will keep a record of your fluid balance and body weight. Your doctor will monitor your blood parameters at regular intervals, particularly electrolytes (e.g., bicarbonate, potassium, magnesium, calcium, and phosphate) and parathyroid hormone and lipid levels.
- If you have high bicarbonate levels in your blood.
- Do not use more solution than prescribed by your doctor. Symptoms of excessive administration include abdominal distension, stomach heaviness, and difficulty breathing.
- Your doctor will regularly check your potassium levels. If they drop too low, you may be given potassium chloride to compensate.
- An inadequate sequence of priming or clamping can produce air perfusion within the peritoneal cavity, which can cause abdominal pain and/or peritonitis.
- Due to a disorder called encapsulating peritoneal sclerosis (EPS), which is a rare and known complication of peritoneal dialysis therapy, you, probably in conjunction with your doctor, should be aware of this possible complication. EPS causes:
- abdominal inflammation (belly)
- thickening of the intestines that may be associated with abdominal pain, abdominal distension, or vomiting. EPS can be fatal.
Children
If you are under 18 years of age, your doctor will carefully evaluate the benefit-risk ratio of using this product.
Using other medicines and PHYSIONEAL 35
- Tell your doctor if you are using, have recently used, or might use any other medicines.
- If you use other medicines, your doctor may need to increase your dose since peritoneal dialysis treatment increases the elimination of certain medicines.
- Be careful if you use heart medications called cardiac glycosides (e.g., digoxin), as you may:
- need potassium and calcium supplements
- develop heart rhythm disturbances (arrhythmia).
- Your doctor will closely monitor you during treatment, especially your potassium levels.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine. The use of Physioneal is not recommended during pregnancy or breastfeeding unless your doctor advises you to do so.
Driving and using machines
This treatment can cause weakness, blurred vision, or dizziness. Do not drive or use machines if you are affected.
3. How to use PHYSIONEAL 35
PHYSIONEAL 35 should be administered into your peritoneal cavity. This cavity is located in the abdomen (belly) between the skin and the peritoneum. The peritoneum is the membrane that surrounds the internal organs, such as the intestines or liver.
Do not use intravenously.
Follow exactly the administration instructions for this medicine indicated by your specialized medical team for peritoneal dialysis. If in doubt, consult them again.
If the bag is damaged, it should be discarded.
Dose and frequency
Your doctor will indicate the appropriate glucose concentration and the number of bags you should use each day.
Use in children and adolescents
If you are under 18 years of age, your doctor will carefully evaluate the prescription of this medicine.
If you interrupt treatment with PHYSIONEAL 35
Do not interrupt peritoneal dialysis without your doctor's consent. Stopping treatment can have harmful consequences for your life.
Method of administration
Before use,
- Warm the bag to 37°C. Use the specially designed heating plate for this purpose. Never submerge the bag in water to warm it. Never use a microwave oven to warm the bag.
- You must use an aseptic technique throughout the administration of the solution, as you have been taught.
- Before performing an exchange, make sure to wash your hands and the area where you will perform the exchange.
- Before opening the overbag, check that it is the correct solution, the expiration date, and the quantity (volume). Lift the bag to check for leaks (excess liquid in the overbag). Do not use the bag if you find any leaks.
- After removing the overbag, check for signs of leaks in the packaging by firmly pressing the bag. Check that the long and short seals are not open at any point. Discard the bag if either seal is open, even partially. Do not use the bag if you detect any leaks.
- Check that the solution is clear. Do not use the bag if the solution is cloudy or contains particles.
- Before starting the exchange, make sure all connections are secure.
- Mix the two chambers carefully by breaking the long seal first and then the short seal (Safety Moon).
- Consult your doctor if you have any questions or doubts about this product or its use.
- Use each bag only once. Discard any unused solution.
- The solution must be administered within 24 hours after mixing.
After use, check that the drainage fluid is not cloudy.
Compatibility with other medicines
Your doctor may prescribe other injectable medicines to be added directly to the PHYSIONEAL 35 bag. In this case, add the medicine through the medication addition site located in the large chamber, before opening the long seal. Disinfect the medication addition site immediately before proceeding with the injection. Use the product immediately after adding the medicine. Consult your doctor if you are unsure.
If you use more PHYSIONEAL 35 bags in 24 hours than you should
If you are administered an excessive dose of PHYSIONEAL 35, you may experience:
- abdominal distension
- stomach heaviness and/or
- difficulty breathing.
Contact your doctor immediately. They will inform you of what to do.
If you have any other questions about the use of this product, ask your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you experience any of the following situations, contact your doctor or peritoneal dialysis unit immediately:
- Hypertension (blood pressure above normal levels).
- Inflammation of the ankles or legs, swollen eyes, difficulty breathing, or chest pain (hypervolemia).
- Abdominal pain.
- Chills (flu-like symptoms), fever,
- Inflamed peritoneum (peritonitis)
These are serious side effects. You may need urgent medical attention.
If you experience any side effect, tell your doctor or peritoneal dialysis unit. This includes any possible side effects not listed in this leaflet.
Frequent (may affect up to 1 in 10 people):
- Changes in your blood parameters:
- increased calcium levels (hypercalcemia)
- decreased potassium levels (hypokalemia) that may cause muscle weakness, muscle contractions, or heart rhythm disturbances.
- Weakness, fatigue.
- Fluid retention (edema).
- Weight gain.
Uncommon (may affect up to 1 in 100 people):
- Decreased fluid removal during dialysis.
- Fainting, dizziness, or headache.
- Cloudy solution extracted from the peritoneum, stomach pain.
- Peritoneal bleeding, pus, swelling, or pain around the catheter exit site, and catheter blockage.
- Nausea, loss of appetite, indigestion, flatulence (gas), thirst, and dry mouth.
- Abdominal distension or inflammation, shoulder pain, abdominal cavity hernia (bulge in the groin).
- Changes in your blood parameters:
- lactic acidosis
- increased carbon dioxide levels
- increased sugar levels (hyperglycemia)
- increased white blood cell count (eosinophilia).
- Difficulty sleeping.
- Low blood pressure (hypotension).
- Cough.
- Muscle or bone pain.
- Facial or throat inflammation.
- Skin rash.
Other side effects related to the peritoneal dialysis procedure:
- Infection around the catheter exit site, catheter blockage.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of PHYSIONEAL 35
Keep this medicine out of the sight and reach of children.
Do not store at a temperature below 4°C.
Do not use this medicine after the expiration date. The expiration date is stated on the label of the outer packaging and on the bag after the abbreviation EXP and the symbol?. The expiration date is the last day of the month indicated.
Discard PHYSIONEAL 35 as instructed.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and any unused medicines. This will help protect the environment.
6. Container contents and additional information
This leaflet does not contain all the information about this medicine. If you have any doubts or are unsure about something, ask your doctor.
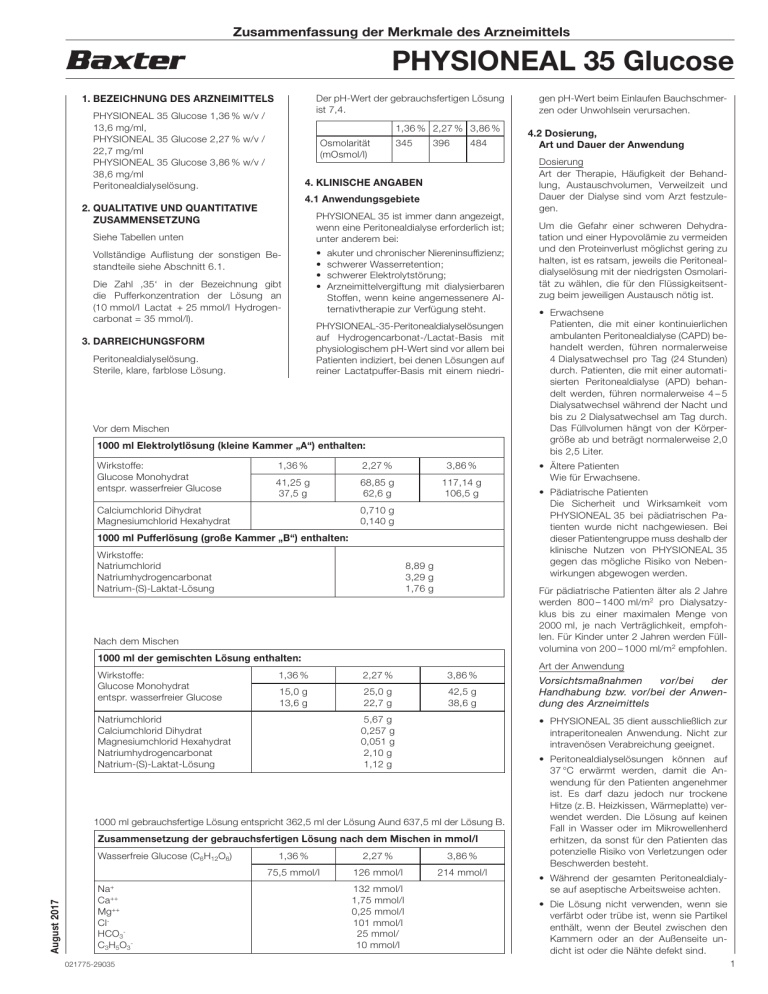
Composition of PHYSIONEAL 35
The mixed peritoneal dialysis solution contains the following active principles:
1.36% | 2.27% | 3.86% | |
Glucose monohydrate (g/l) | 15.0 | 25.0 | 42.5 |
Equivalent to anhydrous glucose (g/l) | 13.6 | 22.7 | 38.6 |
Sodium chloride (g/l) | 5.67 | ||
Calcium chloride dihydrate (g/l) | 0.257 | ||
Magnesium chloride hexahydrate (g/l) | 0.051 | ||
Sodium bicarbonate (g/l) | 2.10 | ||
Solution of (S)-sodium lactate equivalent to (S)-sodium lactate (g/l) | 1.12 |
The other components are: water for injectable preparations, sodium hydroxide, and hydrochloric acid.
The composition in mmol/l of the mixed solution is:
1.36% | 2.27% | 3.86% | |
Anhydrous glucose (mmol/l) | 75.5 | 126 | 214 |
Sodium (mmol/l) Calcium (mmol/l) Magnesium (mmol/l) Chloride (mmol/l) Bicarbonate (mmol/l) Lactate (mmol/l) | 132 1.75 0.25 101 25 10 |
Appearance of the medicine and container contents
PHYSIONEAL 35 is a colorless, transparent, and sterile solution for peritoneal dialysis.
PHYSIONEAL 35 is packaged in a PVC-free plastic bag with two chambers. The two chambers are separated by non-permanent seals. You should only administer PHYSIONEAL 35 when the solutions from the two chambers are fully mixed. Only then should you open the short seal (Safety Moon).
Each bag is wrapped in an overbag and supplied in a cardboard box.
Volume | Number of units per box | Product presentation | Types of connectors |
1.5 l | 5 / 6 | Single bag (DPA) | luer |
1.5 l | 5 / 6 | Double bag (DPCA) | luer |
2.0 l | 4 / 5 | Single bag (DPA) | luer |
2.0 l | 4 / 5 | Double bag (DPCA) | luer |
2.5 l | 3 / 4 | Single bag (DPA) | luer |
2.5 l | 3 / 4 | Double bag (DPCA) | luer |
3.0 l | 3 | Single bag (DPA) | luer |
3.0 l | 3 | Double bag (DPCA) | luer |
4.5 l | 2 | Single bag (DPA) | luer |
5.0 l | 2 | Single bag (DPA) | luer / luer and Line equipment with luer connection for DPA with HomeChoice |
Only some pack sizes may be marketed.
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
Marketing authorization holder
Vantive Health, S.L.
Polígono Industrial Sector 14
C/Pouet de Camilo 2
46394 Ribarroja del Turia (Valencia)
Spain
Manufacturer
Vantive Manufacturing Limited Moneen Road Castlebar County Mayo FR23 XR63 Ireland or Bieffe Medital SpA, Via Nuova Provinciale 23034 Grosotto Italy |
This medicine is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Germany, Austria, Belgium, Bulgaria, Cyprus, Croatia, Denmark, Slovakia, Slovenia, Spain, Estonia, Finland, France, Greece, Netherlands, Hungary, Ireland, Iceland, Latvia, Lithuania, Luxembourg, Malta, Norway, Poland, Portugal, United Kingdom (Northern Ireland), Czech Republic, Romania, Sweden: PHYSIONEAL 35 CLEAR-FLEX
Italy: FIXIONEAL 35
Date of the last revision of this leaflet: April 2022
Detailed information about this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
Vantive, Physioneal, and Clear-Flex are registered trademarks of Vantive Health Inc. or its subsidiaries
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to PHYSIONEAL 35 2.27% GLUCOSE SOLUTION FOR PERITONEAL DIALYSISDosage form: PERITONEAL DIALYSIS, 1000 mlActive substance: Hypertonic solutionsManufacturer: Fresenius Medical Care Deutschland GmbhPrescription requiredDosage form: PERITONEAL DIALYSIS, 1000 mlActive substance: Hypertonic solutionsManufacturer: Fresenius Medical Care Deutschland GmbhPrescription requiredDosage form: PERITONEAL DIALYSIS, 4.25 %Active substance: Hypertonic solutionsManufacturer: Fresenius Medical Care Deutschland GmbhPrescription required
Online doctors for PHYSIONEAL 35 2.27% GLUCOSE SOLUTION FOR PERITONEAL DIALYSIS
Discuss questions about PHYSIONEAL 35 2.27% GLUCOSE SOLUTION FOR PERITONEAL DIALYSIS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















