
PARACETAMOL B.BRAUN 10 mg/ml SOLUTION FOR INFUSION

How to use PARACETAMOL B.BRAUN 10 mg/ml SOLUTION FOR INFUSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
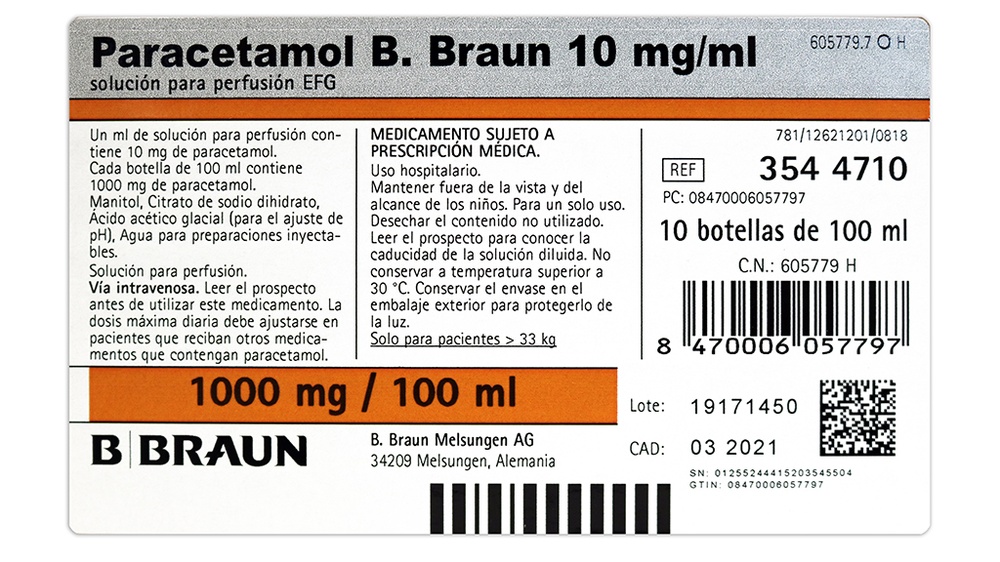
Paracetamol B. Braun 10 mg/ml solution for infusion EFG
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Paracetamol B. Braun is and what it is used for
- What you need to know before you use Paracetamol B. Braun
- How to use Paracetamol B. Braun
- Possible side effects
- Storage of Paracetamol B. Braun
- Contents of the pack and other information
1. What Paracetamol B. Braun is and what it is used for
This medicine is a pain reliever (analgesic) and a fever reducer (antipyretic).
It is used for
- the short-term treatment of moderate pain, especially after surgery;
- the short-term treatment of fever.
2. What you need to know before you use Paracetamol B. Braun
Do not use Paracetamol B. Braun
- if you are allergic to paracetamol or any of the other ingredients of this medicine (listed in section 6);
- if you are allergic (hypersensitive) to propacetamol (another pain reliever that is converted to paracetamol in your body);
- if you have severe liver disease.
Warnings and precautions
Consult your doctor before you start receiving Paracetamol B. Braun.
Be particularly careful with Paracetamol B. Braun
- if you have severe liver or kidney disease or chronic alcoholism;
- if you are taking other medicines that contain paracetamol. In this case, your doctor will adjust the dose;
- in cases of nutritional problems (states of malnutrition or undernutrition) or dehydration;
- if you have a genetic disorder of the enzyme glucose-6-phosphate dehydrogenase (favism).
Before treatment, inform your doctor if any of the above conditions apply to you.
During treatment with Paracetamol B. Braun, inform your doctor immediately if you have severe illnesses, such as severe kidney failure or sepsis (when bacteria and their toxins circulate in the blood, causing damage to organs), or if you have malnutrition or chronic alcoholism or if you are also taking flucloxacillin (an antibiotic). A serious disease called metabolic acidosis (an anomaly in the blood and fluids) has been reported in patients in these situations when paracetamol is used at regular doses for a prolonged period or when paracetamol is taken with flucloxacillin. The symptoms of metabolic acidosis may include: severe breathing difficulties with deep and rapid breathing, drowsiness, feeling unwell (nausea) and vomiting.
Prolonged or frequent use of paracetamol is strongly discouraged. It is recommended that this medicine be used only until you can take analgesics orally again.
Your doctor will make sure that you do not receive a higher dose than recommended, as this may cause serious liver damage.
Other medicines and Paracetamol B. Braun
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
This is especially important if you are taking:
- a medicine called probenecid(used to treat gout): in this case, the dose of paracetamol may need to be reduced;
- analgesics that contain salicylamide: in this case, the dose may need to be adjusted;
- medicines that activate liver enzymes: in these cases, it is necessary to strictly control the dose of paracetamol to avoid liver damage;
- medicines to thin the blood(anticoagulants): in this case, more frequent monitoring of the effect of these medicines may be necessary.
- flucloxacillin(antibiotic), due to a serious risk of alteration of the blood and fluids (called metabolic acidosis) that must be treated urgently (see section 2).
This medicine contains paracetamol, so this should be taken into account if you are taking other medicines that contain paracetamol or propacetamolto avoid an overdose (see section 3).
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine.
- Pregnancy
In case of need, Paracetamol B. Braun can be used during pregnancy. You should use the lowest possible dose that reduces the pain or fever and use it for the shortest possible time. Contact your doctor if the pain or fever does not decrease or if you need to take the medicine more frequently.
- Breastfeeding
Paracetamol B. Braun can be used during breastfeeding.
Paracetamol B. Braun contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per vial; this is essentially “sodium-free”.
3. How to use Paracetamol B. Braun
The recommended dose is:
Your doctor will adjust the dose individually for you based on your body weight and clinical situation.
Method of administration
The doctor will administer this medicine to you through a drip in a vein (intravenously). This process takes about 15 minutes. During the infusion and, especially, towards the end of it, you will be under close supervision.
If you feel that the effect of Paracetamol B. Braun solution for infusion is too strong or too weak, tell your doctor.
If you receive more Paracetamol B. Braun than you should
This is unlikely to happen, as the medicine will be administered by healthcare personnel.
Your doctor will make sure that you do not receive a higher dose than recommended.
However, in case you are given more medicine than you should, symptoms usually appear within the first 24 hours and include: feeling dizzy, nausea, loss of appetite, paleness, and abdominal pain. These symptoms could reflect liver damage.
If you think you may have received a higher dose than you should, tell your doctor immediately. In case of overdose, you should consult a doctor immediately, even if you feel well, to avoid the risk of serious and irreversible liver damage. If necessary, an antidote will be administered.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects may be serious. If you experience any of them, stop using Paracetamol B. Braun and tell your doctor immediately.
Very rare (may affect up to 1 in 10,000 people)
- allergic reactions of varying severity, ranging from skin rash such as hives to anaphylactic shock;
- severe skin reactions
- abnormal decrease in the levels of some types of blood cells (platelets, white blood cells) may occur.
Other possible side effects:
Rare (may affect up to 1 in 1,000 people)
- changes in laboratory test results: abnormally high levels of liver enzymes when blood tests are performed;
- low blood pressure;
- general feeling of being unwell or weakness
Very rare (may affect up to 1 in 10,000 people)
- Very rare cases of severe skin reactions have been reported
Frequency not known (cannot be estimated from the available data)
- redness of the skin, flushing or itching;
- increased heart rate;
- a serious disease that can make the blood more acidic (called metabolic acidosis) in patients with severe illness who use paracetamol (see section 2).
During clinical trials, the frequent appearance of side effects at the injection site (feeling of pain or burning) has been observed.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Paracetamol B. Braun
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton after EXP. The expiry date refers to the last day of the month shown.
Do not store above 30°C.
Keep the vial in the outer packaging to protect it from light.
6. Container Content and Additional Information
Composition of Paracetamol B. Braun:
The active ingredient is paracetamol.
One ml contains 10 mg of paracetamol.
Each 10 ml ampoule contains 100 mg of paracetamol.
Each 50 ml vial contains 500 mg of paracetamol.
Each 100 ml vial contains 1000 mg of paracetamol.
The other components are:
Mannitol, sodium citrate dihydrate, glacial acetic acid (for pH adjustment), water for injectable preparations.
Appearance of the Product and Container Content
Paracetamol B. Braun solution for infusion is a clear and colorless to slightly pink-orange solution. Perception may vary.
Paracetamol B. Braun is packaged in 50 ml and 100 ml plastic vials or a 10 ml plastic ampoule.
Package sizes: 20 × 10 ml, 10 × 50 ml, 10 × 100 ml
Only some package sizes may be marketed.
Marketing Authorization Holder
- Braun Melsungen AG,
Address:
Carl-Braun-Straße 1, Postal Address:
34212 Melsungen, Germany 34209 Melsungen, Germany
Phone: +49/5661/71-0
Fax: +49/5661/71-4567
Manufacturer
- Braun Medical S. A.
Carretera de Terrassa 121
08191 Rubí (Barcelona), Spain
This medicinal product is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Bulgaria, Slovakia, Estonia, Finland, France, Italy, Luxembourg, Netherlands, Portugal, Czech Republic, Sweden | Paracetamol B. Braun 10 mg/ml |
Belgium | Paracetamol B. Braun 10 mg/ml solution for infusion, oplossing voor infusie, Infusionslösung |
Austria, Germany | Paracetamol B. Braun 10 mg/ml Infusionslösung |
Lithuania | Paracetamol B. Braun 10 mg/ml infuzinis tirpalas |
Latvia | Paracetamol B. Braun 10 mg/ml šķīdums infuzijām |
Romania | Paracetamol B. Braun 10 mg/ml soluție perfuzabilă |
Slovenia | Paracetamol B. Braun 10 mg/ml raztopina za infundiranje |
Spain | Paracetamol B. Braun 10 mg/ml solution for infusion |
Ireland, United Kingdom (Northern Ireland), Malta | Paracetamol 10 mg/ml solution for infusion |
Denmark, Norway, Poland | Paracetamol B. Braun |
Date of the Last Revision of this Leaflet: 01/2025
Other Sources of Information
Detailed information on this medicinal product is available on the website ofthe Spanish Agency for Medicines and Health Products.//www.aemps/gob.es/
----------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals.
Dosage
- The 100 ml polyethylene vialis restricted to adults, adolescents, and children weighing more than 33 kg.
- The 50 ml polyethylene vialis restricted to full-term newborns, infants, and children weighing more than 10 kg and up to 33 kg.
- The 10 ml polyethylene ampouleis restricted to full-term newborns and children from 28 days to less than 24 months weighing up to 10 kg.
The administered volume must not exceed the determined dose.If necessary, before administration, it is necessary to dilute the desired volume in a suitable infusion solution (see below "Method of Administration and Dilution"), or use an automatic syringe.
RISK OF MEDICATION ERRORS Be careful to avoid administration errors due to possible confusion between milligrams (mg) and milliliters (ml), which could lead to accidental overdose and death. |
Prolonged or frequent use of paracetamol is strongly discouraged. It is recommended that this medicinal product be used only until oral analgesics can be taken again.
Dose based on patient weight (please consult the dosage table included below)
10 ml Ampoule | ||||
Patient Weight | Dose per Administration | Volume per Administration | Maximum Volume of Paracetamol B. Braun (10 mg/ml) per Administration, based on the Upper Limits of the Weight Group (ml)*** | Maximum Daily Dose** |
?10 kg* | 7.5 mg/kg | 0.75 ml/kg | 7.5 ml | 30 mg/kg |
50 ml Vial | ||||
Patient Weight | Dose per Administration | Volume per Administration | Maximum Volume of Paracetamol B. Braun (10 mg/ml) per Administration, based on the Upper Limits of the Weight Group (ml)*** | Maximum Daily Dose** |
> 10 kg up to ? 33 kg | 15 mg/kg | 1.5 ml/kg | 49.5 ml | 60 mg/kg Not exceeding 2 g |
100 ml Vial | ||||
Patient Weight | Dose (per Administration) | Maximum Volume per Administration | Maximum Volume of Paracetamol B. Braun (10 mg/ml) per Administration, based on the Upper Limits of the Weight Group (ml)*** | Maximum Daily Dose** |
> 33 kg up to ? 50 kg | 15 mg/kg | 1.5 ml/kg | 75 ml | 60 mg/kg Not exceeding 3 g |
> 50 kg with additional risk factors for hepatotoxicity | 1 g | 100 ml | 100 ml | 3 g |
> 50 kg and without additional risk factors for hepatotoxicity | 1 g | 100 ml | 100 ml | 4 g |
*Preterm newborns:
There are no safety and efficacy data available for preterm newborns.
**Maximum daily dose:
The maximum daily dose indicated in the table above refers to patients who are not receiving other medicinal products containing paracetamol. The dose should be adjusted taking into account these other medicinal products.
***Patients with lower weight will require smaller volumes
The minimum interval between each administration must be at least 4 hours.
The minimum interval between each administration in patients with severe renal impairment must be at least 6 hours.
No more than 4 doses should be administered in 24 hours.
Severe renal impairment:
In case paracetamol needs to be administered to patients with severe renal impairment (creatinine clearance ≤ 30 ml/min), it is recommended to reduce the dose and increase the minimum interval between each administration to 6 hours.
Adults with hepatocellular insufficiency, chronic alcoholism, chronic malnutrition (low hepatic glutathione reserves), or dehydration:
The maximum daily dose should not exceed 3 g (see section "Warnings and Precautions").
Method of Administration and Dilution
Paracetamol B. Braun can be diluted up to one-tenth (one volume of Paracetamol B. Braun in nine volumes of diluent) in a 9 mg/ml (0.9%) sodium chloride infusion solution or in a 50 mg/ml (5%) glucose solution or a combination of both solutions.
For single use. The medicinal product must be used immediately after opening. Discard the unused solution.
As with all infusion solutions presented in containers with air spaces inside, it should be remembered that close monitoring is required, particularly at the end of the infusion, regardless of the route of administration. This monitoring at the end of the infusion applies particularly to central venous infusions, in order to avoid gas embolism.
Shelf Life after Opening the Container
The infusion should be started immediately after connecting the container to the administration equipment.
Shelf Life after Dilution
The chemical and physical stability of the product has been demonstrated (including infusion time) for 48 hours at 23 °C.
From a microbiological point of view, the medicinal product should be administered immediately. If not used immediately, the in-use storage times and conditions are the responsibility of the user.
The solution should be visually inspected for particles or changes/alterations in coloration before administration. Do not use if the solution is not transparent and colorless to slightly pink-orange (perception may vary), or if the container or its closure is damaged or shows any visible signs of deterioration.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to PARACETAMOL B.BRAUN 10 mg/ml SOLUTION FOR INFUSIONDosage form: TABLET, 1 gActive substance: paracetamolManufacturer: Uxa Farma S.A.Prescription requiredDosage form: TABLET, 1 gActive substance: paracetamolManufacturer: Laboratorios Cinfa S.A.Prescription not requiredDosage form: TABLET, 1 gActive substance: paracetamolManufacturer: Laboratorios Cinfa S.A.Prescription not required
Online doctors for PARACETAMOL B.BRAUN 10 mg/ml SOLUTION FOR INFUSION
Discuss questions about PARACETAMOL B.BRAUN 10 mg/ml SOLUTION FOR INFUSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















