
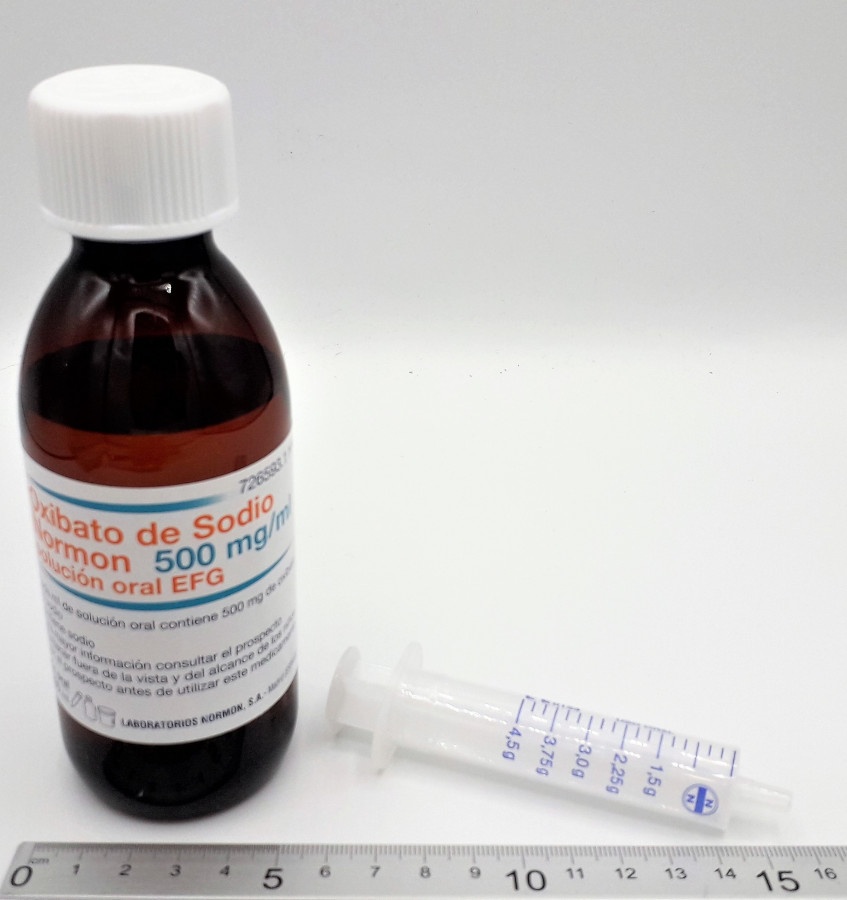
OXIBATO SODICO SALA 500 mg/mL ORAL SOLUTION

How to use OXIBATO SODICO SALA 500 mg/mL ORAL SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Oxibate Sodium Sala 500 mg/ml Oral Solution EFG
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What Oxibate Sodium Sala is and what it is used for
- What you need to know before you take Oxibate Sodium Sala
- How to take Oxibate Sodium Sala
- Possible side effects
- Storing Oxibate Sodium Sala
- Contents of the pack and other information
1. What Oxibate Sodium Sala is and what it is used for
Oxibate Sodium Sala contains the active substance oxibate sodium. Oxibate Sodium Sala works by consolidating nighttime sleep, although its exact mechanism of action is unknown.
Oxibate Sodium Sala is used to treat narcolepsy with cataplexy in adults, adolescents, and children from 7 years of age.
Narcolepsy is a sleep disorder that can include sleep attacks during the hours when you are normally awake, as well as cataplexy, sleep paralysis, hallucinations, and insomnia. Cataplexy is the sudden onset of weakness or muscle paralysis without loss of consciousness, in response to a sudden emotional reaction such as anger, fear, joy, laughter, or surprise.
2. What you need to know before you take Oxibate Sodium Sala
Do not take Oxibate Sodium Sala
-if you are allergic to oxibate sodium or any of the other ingredients of this medicine (listed in section 6);
- -if you have a deficiency of succinic semialdehyde dehydrogenase (a rare metabolic disorder);
-if you suffer from severe depression;
-if you are receiving treatment with opioid or barbiturate medications.
Warnings and precautions
Consult your doctor or pharmacist before starting to take Oxibate Sodium Sala.
- -if you have respiratory or pulmonary problems (and especially if you are obese), as Oxibate Sodium Sala has the potential to cause breathing difficulties;
- -if you have or have had depression, suicidal thoughts, anxiety, psychosis (a mental disorder that can involve hallucinations, incoherent speech, or disorganized and agitated behavior), or bipolar disorder;
- -if you have heart failure, hypertension (high blood pressure), liver or kidney problems, you may need to have your dose adjusted;
- -if you have previously used drugs or abused medications;
- -if you suffer from epilepsy, as the use of Oxibate Sodium Sala is not recommended in this disease;
- -if you have porphyria (a rare metabolic disorder).
If you have any of these problems, inform your doctor before taking Oxibate Sodium Sala.
If, while taking Oxibate Sodium Sala, you experience nocturnal urine loss and incontinence (both urinary and fecal), confusion, hallucinations, sleepwalking episodes, or abnormal thinking, you should immediately inform your doctor. Although these effects are rare, if they appear, they are usually mild to moderate in nature.
In elderly patients, the doctor will carefully monitor their progress to check if this medicine produces the desired effects.
Oxibate Sodium Sala has a well-known potential for abuse. There have been cases of dependence after illicit use of oxibate sodium.
Your doctor will ask you if you have used any drugs before starting to take this medicine and while you are taking this medicine.
Children and adolescents
Oxibate Sodium Sala can be taken by adolescents and children from 7 years of age who weigh more than 15 kg.
Oxibate Sodium Sala cannot be taken by children under 7 years of age or who weigh less than 15 kg.
If you are a child or adolescent, your doctor will regularly check your body weight.
While the doctor is adjusting the dose, which can take several weeks, parents/caregivers should carefully monitor the child's breathing during the first 2 hours after taking oxibate sodium to assess if there is any anomaly in breathing; for example, interruption of breathing during short periods while sleeping, noisy breathing, and a bluish color on the lips and face. If breathing anomalies are observed, medical assistance should be sought and the doctor should be informed as soon as possible. If any anomaly is observed after the first dose, the second dose should not be administered. If no anomaly is observed, the second dose can be administered. The second dose should not be administered before 2.5 hours or after 4 hours after the administration of the first dose.
If you have had or are having unpleasant feelings, especially if you feel very sad or have lost interest in life, it is important that you inform your doctor or caregiver.
Using Oxibate Sodium Sala with other medicines
Inform your doctor or pharmacist if you are using, have recently used, or may need to use any other medicine.
In particular, Oxibate Sodium Sala should not be used with medicines that induce sleep and medicines that reduce the activity of the Central Nervous System (the Central Nervous System is the part of the body composed of the brain and spinal cord):
You should also inform your doctor or pharmacist if you are using any of the following types of medicines:
- medicines that increase the activity of the central nervous system
- antidepressants
- medicines that can be metabolized in a similar way by the body (e.g., valproate, phenytoin, or ethosuximide, which are used to treat epileptic seizures)
- topiramate (used to treat epilepsy)
If you are taking valproate, your daily dose of Oxibate Sodium Sala will need to be adjusted (see section 3) as it may lead to interactions.
Taking Oxibate Sodium Sala with food, drinks, and alcohol
You should not drink alcohol while taking this medicine, as its effects may be increased.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine. There have been very few women who have taken oxibate sodium during pregnancy, and some of them suffered spontaneous abortions. The risk of taking Oxibate Sodium Sala during pregnancy is not known, so its use is not recommended in pregnant women or women trying to become pregnant.
Patients taking Oxibate Sodium Sala should interrupt breastfeeding, as Oxibate Sodium Sala passes into breast milk.
Changes in sleep have been observed in infants of mothers exposed to Oxibate Sodium Sala.
Driving and using machines
Oxibate Sodium Sala may affect you if you drive or use machines. Do not drive, do not use heavy machinery, or perform any activity that may be hazardous or requires mental alertness for at least 6 hours after taking Oxibate Sodium Sala. When you first start taking Oxibate Sodium Sala and until you know if it makes you sleepy the next day, be especially careful when driving, operating heavy machinery, or doing any other activity that could be hazardous or requires complete mental alertness.
In pediatric patients, doctors, parents, or caregivers are warned that the waiting time to perform activities that require a state of mental alertness, motor coordination, or activities that may have a physical risk may be more than 6 hours, depending on individual sensitivity.
Oxibate Sodium Sala contains sodium
This medicine contains 182.24 mg of sodium (the main component of table/cooking salt) per gram. This is equivalent to 9.11% of the maximum daily recommended sodium intake for an adult.
Consult your doctor or pharmacist if you need 2 grams of oxibate sodium or more per day for a prolonged period, especially if you have been recommended a low-salt (sodium) diet.
3. How to take Oxibate Sodium Sala
Follow exactly the administration instructions of this medicine indicated by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again.
It is important that you only use the syringe included in the box during the preparation of the doses of Oxibate Sodium Sala.
Adults: taking Oxibate Sodium Sala alone
- For adults, the recommended initial dose is 4.5 grams each day, divided into two separate doses of 2.25 grams.
- Your doctor may gradually increase your dose up to a maximum of 9 grams each day, divided into two separate doses of 4.5 grams.
- Take Oxibate Sodium Sala orally twice each night:
- Take the first dose when going to bed and the second dose 2½ to 4 hours later. You may need an alarm clock to make sure you wake up to take the second dose.
- Food reduces the amount of Oxibate Sodium Sala absorbed by your body. Therefore, it is best to take Oxibate Sodium Sala always at a certain time, 2 or 3 hours after meals.
- Prepare both doses before going to bed.
- Take the doses within 24 hours after preparation.
Adolescents and children from 7 years old who weigh 15 kg or more: taking Oxibate Sodium Sala alone
For children from 7 years old who weigh 15 kg or more, the doctor will calculate the suitable dose based on body weight.
The doctor will calculate the suitable dose for you. Do not exceed the dose that has been prescribed for you.
Adults: taking Oxibate Sodium Sala with valproate
If you are taking valproate with Oxibate Sodium Sala, your doctor will adjust the dose of Oxibate Sodium Sala.
- For adults, the recommended initial dose of Oxibate Sodium Sala when used with valproate is 3.6 grams each day, divided into two separate doses of 1.8 grams.
- Take the first dose when going to bed and the second dose 2½ to 4 hours later.
Adolescents and children from 7 years old who weigh 15 kg or more: taking Oxibate Sodium Sala with valproate
If you are taking valproate with Oxibate Sodium Sala, your doctor will adjust the dose of Oxibate Sodium Sala.
Liver or kidney problems
If you have kidney problems, you should take into account the dietary recommendations to reduce sodium intake (salt). If you have liver problems, the initial dose should be reduced by half. Your doctor may gradually increase your dose.
Instructions for diluting Oxibate Sodium Sala
The following instructions explain how to prepare Oxibate Sodium Sala. Read the instructions carefully and follow them step by step. Do not allow children to prepare Oxibate Sodium Sala.
To help you, the Oxibate Sodium Sala pack contains 1 bottle of medicine, a graduated syringe, and two dosing cups with child-resistant caps.
Step 1
- Remove the cap from the bottle by pressing down and twisting counterclockwise (to the left).
- After removing the cap, place the bottle upright on a table.
Keeping the bottle in a vertical position, insert the pressure adapter into the neck of the bottle. This should only be done the first time the bottle is opened. The adapter can be left on the bottle for subsequent uses.
Step 2
- Next, insert the tip of the graduated syringe into the center of the bottle opening and press firmly (See Figure 1).
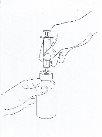 Figure 1
Figure 1
- Keeping the bottle and syringe in one hand, turn the bottle upside down and withdraw the prescribed dose with the other hand by pulling the plunger. NOTE: the medicine will not flow into the syringe unless you keep the bottle in an inverted position (see Figure 2).
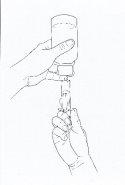 Figure 2
Figure 2
Step 3
- Place the bottle in a vertical position. Remove the syringe from the center of the bottle opening.
- Empty the medicine from the syringe into one of the provided dosing cups by pushing the plunger (See Figure 3). Repeat this step for the second dosing cup.
- Then add approximately 60 ml of water to each dosing cup (60 ml is approximately 4 tablespoons).
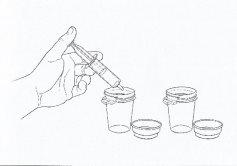 Figure 3
Figure 3
Step 4
- Put the caps on the dosing cups and turn each cap clockwise (to the right) until you feel the click and close it in the child-resistant position (See Figure 4).
- Rinse the syringe with water.
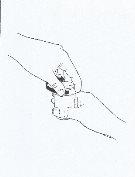 Figure 4
Figure 4
Just before going to bed:
- Adult patients should place their second dose near their bed.
Parents or caregivers of adolescents and children from 7 years old should not leave the second dose near the child's bed or within their reach.
- You may need an alarm clock to make sure you wake up to take your second dose, no earlier than 2.5 hours and no later than 4 hours after your first dose.
Then:
- Remove the cap from the first dosing cup by pressing on the child-resistant cap and twisting counterclockwise (to the left).
- Drink the first dose sitting in bed, replace the cap, and then lie down immediately. In the case of children who sleep more than 8 hours but less than 12, the first dose can be administered after the child has slept for 1 to 2 hours.
- When you wake up or wake the child between 2 ½ and 4 hours later, remove the cap from the second dosing cup. Sitting in bed, drink the second dose just before going back to sleep. Replace the second cap.
If you think the effect of Oxibate Sodium Sala is too strong or too weak, tell your doctor or pharmacist.
If you take more Oxibate Sodium Sala than you should
The symptoms of an overdose of Oxibate Sodium Sala may include agitation, confusion, altered mobility, breathing difficulties, blurred vision, excessive sweating, headache, vomiting, decreased consciousness that can lead to coma and epileptic seizures, excessive thirst, muscle cramps, and weakness. If you take more of this medicine than you should, or take it by accident, seek immediate emergency medical help. You should take the medicine box with you, even if it is empty.
If you forget to take Oxibate Sodium Sala
If you forget to take the first dose, take it as soon as you remember and continue with the procedure described previously. If you miss the second dose, skip that dose and do not take Oxibate Sodium Sala again until the next night. Do not take a double dose to make up for the missed doses.
If you are not sure if you have taken Oxibate Sodium Sala
In case of doubt about the administration of a dose, do not re-administer the dose to reduce the risk of overdose.
If you stop taking Oxibate Sodium Sala
You should continue taking Oxibate Sodium Sala while your doctor continues to prescribe it for you. If the medication is interrupted, cataplexy attacks may return, and you may experience insomnia, headache, anxiety, dizziness, sleep disorders, somnolence, hallucinations, and abnormal thinking.
If you interrupt treatment with this medicine for more than 14 days, you should consult your doctor, as you may need to restart treatment from a lower dose.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them. These are often of mild to moderate intensity.
Adults: most frequent adverse effects observed in clinical studies(occurring in 10% to 20% of patients):
- dizziness
- nausea
- headache.
If you experience any of these adverse effects, inform your doctor immediately.Children and adolescents: most frequent adverse effects observed in a clinical study:
- bedwetting (18.3%)
- nausea (12.5%)
- vomiting (8.7%)
- weight loss (8.7%)
- decreased appetite (6.7%)
- headache (5.8%)
- dizziness (5.8%)
- suicidal thoughts (1%)
- feeling mentally unwell (loss of contact with reality) (1%)
If you experience any of these adverse effects, inform your doctor immediately.
The adverse effects in adults and children are the same. If you experience any of these adverse effects, inform your doctor immediately:
Very frequent (may affect more than 1 in 10 people):
- nausea,
- dizziness,
- headache.
Frequent (may affect up to 1 in 10 people):
- sleep problems such as insomnia, abnormal dreams, sleep paralysis, somnolence, nightmares, sleepwalking, bedwetting, excessive daytime sleepiness, difficulty falling asleep in the middle of the night,
- feeling of drunkenness, tremors, confusion or disorientation, blurred vision, balance disorder, falls, feeling of "dizziness" (vertigo),
- feeling heartbeats, increased blood pressure, shortness of breath
- vomiting, stomach pain, diarrhea
- anorexia, decreased appetite, weight loss
- weakness, fatigue, sedation
- sweating
- depression
- muscle cramps, swelling
- joint pain, back pain
- attention disorder, sensitivity disorder especially to touch, abnormal touch sensation, abnormal taste
- anxiety, nervousness
- urinary incontinence
- snoring, nasal congestion
- rash
- breast inflammation, nasal and throat inflammation
Infrequent (may affect up to 1 in 100 people):
- psychosis (a mental disorder that can include hallucinations, incoherent speech or
- disorganized and agitated behavior)
- paranoia, abnormal thinking, hallucinations, agitation, attempted suicide
- difficulty falling asleep, restless legs
- memory loss
- myoclonus (involuntary muscle contractions)
- involuntary bowel movements
- hypersensitivity
Frequency not known (cannot be estimated from the available data):
- convulsion
- decrease in depth or frequency of breathing, short cessation of breathing during sleep
- hives
- suicidal thoughts, delirium, thoughts of committing violent acts (including harming others)
- irritability, aggression
- euphoric mood
- panic attack
- mania/bipolar disorder
- dry mouth, dehydration
- facial swelling (angioedema)
- bruxism (bruxism and jaw clenching)
- polyuria/urinary urgency (increased need to urinate)
- tinnitus (noise in the ears, such as ringing or buzzing)
- sleep-related eating disorder
- increased appetite
- loss of consciousness
- dyskinesia (e.g., abnormal and uncontrolled movements of the limbs)
- dandruff
- increased sexual desire
- nocturia (excessive urination at night)
- feeling of suffocation
If you experience any of these side effects, inform your doctor immediately.
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor, pharmacist, or nurse, even if it is a possible adverse effect that is not listed in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es.
By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Oxibato Sodium Sala
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date that appears on the bottle after (CAD). The expiration date is the last day of the month indicated.
After dilution in the dosing cups, the preparation should be used within 24 hours.
Once the Oxibato Sodium Sala bottle is opened, any unused content must be discarded after 90 days.
Medicines should not be thrown away through the sewers or in the trash. Ask your pharmacist how to dispose of the packaging and medicines that are no longer needed. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Oxibato Sodium Sala
-The active ingredient is sodium oxibate. Each ml contains 500 mg of sodium oxibate.
-The other components are purified water, malic acid, and sodium hydroxide.
Appearance of the Product and Package Contents
Oxibato Sodium Sala is presented in a 200 ml amber plastic bottle containing 180 ml of oral solution, closed with a child-resistant cap. Each package contains a bottle, a pressure-resistant adapter (PIBA), a graduated plastic syringe, and two dosing cups with child-resistant caps.
Oxibato Sodium Sala is a clear and colorless solution.
Marketing Authorization Holder and Manufacturer
Laboratorio Reig Jofré, S.A.
Gran Capitan 10,
08970 Sant Joan Despí (Barcelona)
Spain
Your doctor should have given you a package of information about Oxibato Sodium Sala, which includes a booklet on how to take the medicine, a patient information leaflet with Frequently Asked Questions, and a patient alert card.
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
Laboratorio Reig Jofré, S.A.
Gran Capitan 10,
08970 Sant Joan Despí (Barcelona)
Spain
This prospectus was approved in: October 2023
Other Sources of Information
Detailed information about this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS): http://www.aemps.gob.es/.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OXIBATO SODICO SALA 500 mg/mL ORAL SOLUTIONDosage form: ORAL SOLUTION/SUSPENSION, 300 mg/mlActive substance: sodium oxybateManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: ORAL SOLUTION/SUSPENSION, 500 mg/mlActive substance: sodium oxybateManufacturer: Laboratorios Normon S.A.Prescription requiredDosage form: ORAL SOLUTION/SUSPENSION, 500 mg/mlActive substance: sodium oxybateManufacturer: Zentiva K.S.Prescription required
Online doctors for OXIBATO SODICO SALA 500 mg/mL ORAL SOLUTION
Discuss questions about OXIBATO SODICO SALA 500 mg/mL ORAL SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions













