OCTAPLEX, 500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION

How to use OCTAPLEX, 500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
PACKAGE LEAFLET: INFORMATION FOR THE USER
OCTAPLEX500 IU, powder and solvent for solution for infusion. Human prothrombin complex.
OCTAPLEX1000 IU, powder and solvent for solution for infusion. Human prothrombin complex.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information:
- What is Octaplex and what is it used for
- What you need to know before you use Octaplex
- How to use Octaplex
- Possible side effects
- Storage of Octaplex
- Contents of the pack and other information
1. What is Octaplex and what is it used for
Octaplex belongs to a group of medicines called coagulation factors. It contains human coagulation factors II, VII, IX, and X that are dependent on vitamin K.
Octaplex is used to treat and prevent bleeding:
- Caused by medicines called vitamin K antagonists (such as warfarin). These medicines block the effect of vitamin K and cause a deficiency of vitamin K-dependent coagulation factors in your body.
Octaplex is used when a rapid correction of the deficiency is required.
- In people who are born with this deficiency of vitamin K-dependent coagulation factors II and X. It is used when a specific purified coagulation factor product is not available.
2. What you need to know before you use Octaplex
Do not use Octaplex:
- If you are allergic to human prothrombin complex or to any of the other ingredients of this medicine (listed in section 6).
- If you are allergic to heparin or if heparin has ever caused you a reduction in blood platelet count.
- If you have IgA deficiency with known antibodies against IgA.
Warnings and precautions:
- Ask the advice of a doctor with experience in the treatment of blood coagulation disorders when you receive Octaplex.
- If you have an acquired deficiency of vitamin K-dependent coagulation factors (e.g., caused by treatment with vitamin K antagonists), Octaplex can only be used if a rapid correction of the deficiency is necessary, such as in major bleeding or emergency surgery. In other cases, it is usually sufficient to reduce the dose of vitamin K antagonists and/or administer vitamin K.
- If you are receiving a vitamin K antagonist medicine (such as warfarin), you may have a higher risk of forming blood clots. In this case, treatment with Octaplex may increase the risk.
- If you are born with a deficiency of vitamin K-dependent coagulation factors, the specific coagulation factor should be used if available.
- If an allergic reaction or anaphylactic reaction occurs, the infusion of the medicine will be stopped immediately and you will be given appropriate treatment.
- There is a risk of thrombosis or disseminated intravascular coagulation (a serious disease in which blood clots form throughout the body) when you receive Octaplex (especially if you have received it regularly). You will be carefully monitored for signs or symptoms of intravascular coagulation or thrombosis.
This is especially important if you have a history of coronary heart disease, liver disease, if you are going to have surgery, and also if Octaplex is given to very small babies.
- There are no data available on the use of Octaplex in case of bleeding during birth due to vitamin K deficiency in newborns.
Viral safety
- When human blood or plasma-derived medicines are administered, certain measures are taken to prevent the transmission of infections to patients. These measures include careful selection of blood and plasma donors to ensure the exclusion of those at risk of carrying infections and testing for signs of viruses/infections in individual donations and plasma pools. Manufacturers of these products also include processing steps that are capable of inactivating or removing viruses. Despite these measures, when human blood or plasma-derived medicines are administered, the possibility of transmitting an infection cannot be totally excluded. This also applies to all unknown or emerging viruses or other types of infections.
The measures taken are considered effective against enveloped viruses such as human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV). The measures taken may have limited value against non-enveloped viruses, such as hepatitis A virus (HAV) and parvovirus B19. Parvovirus B19 infection can be severe for a pregnant woman (fetal infection) and for subjects with impaired immune systems or suffering from certain types of anemia (e.g., sickle cell disease or hemolytic anemia).
It is strongly recommended that every time you are administered a dose of Octaplex, the name and batch number of the product should be recorded to maintain a link with the batches used.
- It is recommended to be properly vaccinated (hepatitis A and B) if you are administered human prothrombin complex regularly or repeatedly.
Children and adolescents
There are no data available on the use of Octaplex in children and adolescents.
Other medicines and Octaplex
- Octaplex must not be mixed with other medicines.
- Octaplex blocks the effect of vitamin K antagonist medicines (such as warfarin), but no interactions with other medicines are known.
- Octaplex may affect the results of coagulation tests that are sensitive to heparin.
- Tell your doctor or pharmacist if you are taking or have recently taken or might take any other medicines.
Pregnancy and breast-feeding
- Octaplex should only be used during pregnancy and breast-feeding if clearly necessary. Consult your doctor or pharmacist before using any medicine.
Driving and using machines:
Octaplex has not been reported to affect the ability to drive and use machines.
Important information about some of the ingredients of Octaplex
Heparin may cause allergic reactions and a decrease in blood cell count that can affect the blood coagulation system. Patients with a history of heparin-induced allergic reactions should avoid the use of medicines containing heparin.
This medicine contains 75 - 125 mg (vial of 500 IU) or 150 - 250 mg (vial of 1000 IU) of sodium (the main component of cooking/table salt) per vial. This is equivalent to 3.8 - 6.3% or 7.5 - 12.5% of the maximum recommended daily intake of sodium for an adult.
3. How to use Octaplex
Treatment with Octaplex should be supervised by a doctor with experience in the treatment of blood coagulation disorders.
- First, the powder is dissolved in water.
- Then the solution is administered through a vein (intravenously).
The amount of Octaplex you will receive and the duration of your treatment will depend on:
- the severity of your disease
- the location and severity of the bleeding, and
- your general condition.
If you use more Octaplex than you should
In case of overdose, the risk of developing:
- coagulation complications (such as heart attack and blood clots in your veins or lungs)
- disseminated intravascular coagulation (a serious disease in which blood clots form throughout the body)
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Common: (may affect up to 1 in 10 people)
Blood clots in the blood vessels
Uncommon: (may affect up to 1 in 100 people)
Anxiety, increased blood pressure, asthma-like symptoms, coughing up blood, nosebleeds, burning at the injection site, and blood clots in the device.
Rare: (may affect up to 1 in 1,000 people)
Allergic reactions may occur.
A temporary increase in liver test results (transaminases) has rarely been observed.
Patients treated with Octaplex for substitution therapy may develop neutralizing antibodies (inhibitors) against one or more of the coagulation factors contained. If these inhibitors appear, substitution therapy will not be very effective.
Very rare: (may affect up to 1 in 10,000 people)
An increase in temperature (fever) has been observed.
There is a risk of blood coagulation after administration of this medicine.
Frequency not known: (cannot be estimated from the available data)
Severe allergic reaction and shock, hypersensitivity, shivering, heart failure, increased heart rate, failure of blood circulation, decrease in blood pressure, respiratory failure, difficulty breathing, nausea, vomiting, skin rash, chills.
The heparin in this preparation may cause a sudden drop in the number of platelets in the blood. This is an allergic reaction called "heparin-induced thrombocytopenia type II". Rarely, in patients who have not previously been hypersensitive to heparin, this drop in platelet count may occur between 6 and 14 days after the start of treatment. In patients with previous hypersensitivity, this alteration may develop within hours of starting treatment.
Treatment with Octaplex should be stopped immediately in patients who show this allergic reaction. These patients should not receive heparin-containing medicines in the future.
For information on viral safety, see section 2.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Octaplex
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label. The expiry date refers to the last day of the month stated.
Store the vial in the outer packaging to protect it from light and below 25°C. Do not freeze.
The powder should always be dissolved immediately before injection. The stability of the solution has been demonstrated for a maximum of 8 hours at 25°C. However, to prevent contamination, the solution should be used immediately and in a single occasion.
6. Contents of the pack and other information
Composition of Octaplex per vial and after reconstitution with 20 ml (500 IU)/40 ml (1000 IU) of solvent:
The active substances are:
Component name | Octaplex Quantity per 500 IU vial | Octaplex Quantity per 1000 IU vial | OCTAPLEX Quantity per mL of reconstituted solution |
Total proteins: | 260 - 820 mg | 520 - 1640 mg | 13 - 41 mg/mL |
Active substances | |||
Human coagulation factor II | 280 - 760 IU | 560 - 1520 IU | 14 - 38 IU/mL |
Human coagulation factor VII | 180 - 480 IU | 360 - 960 IU | 9 - 24 IU/mL |
Human coagulation factor IX | 500 IU | 1000 IU | 25 IU/mL |
Human coagulation factor X | 360 - 600 IU | 720 - 1200 IU | 18 - 30 IU/mL |
Other active components | |||
Protein C | 260 - 620 IU | 520 - 1240 IU | 13 - 31 IU/mL |
Protein S | 240 - 640 IU | 480 - 1280 IU | 12 - 32 IU/mL |
The specific activity of factor IX is ≥ 0.6 IU/mg protein.
The other components are heparin, trisodium citrate dihydrate, and water for injection.
Appearance and packaging
Octaplex is presented as a powder and solvent for solution for infusion. It is a white or slightly colored hygroscopic powder or a friable mass in a glass vial. The solvent is water for injection and is supplied in a glass vial. The reconstituted solution is clear or slightly opalescent and may be colored.
Octaplex is presented in a carton containing:
- 1 vial with powder for solution for injection.
- 1 vial with solvent, water for injection
- 1 Nextaro transfer device.
Marketing authorisation holder:
Octapharma S.A.
Avda. Castilla, 2. (P.E. San Fernando)
Ed. Dublin – 2ª Planta
28830 San Fernando de Henares
Madrid
Manufacturer:
Octapharma Pharmazeutika Produktionsges.m.b.H.Oberlaaer Str. 2351100 ViennaAustria
or
Octapharma Lingolsheim S.A.S.
72 Rue du Maréchal Foch
67380 Lingolsheim
France
This medicine is authorised in the Member States of the European Economic Area under the following names:
Austria, Belgium, Bulgaria, Cyprus, Croatia, Denmark, Estonia, Finland, France, Germany, Hungary, Iceland, Ireland, Latvia, Lithuania, Luxembourg, Malta, Netherlands, Norway, Poland, Portugal, Republic of Slovenia, Republic of Slovakia, Spain, United Kingdom: Octaplex
Czech Republic, Sweden: Ocplex
Italy and Romania: Pronativ
Date of last revision of this leaflet: 06/2024
INSTRUCTIONS FOR HEALTHCARE PROFESSIONALS
General information on how to use Octaplex is given in section 3.
The following information is intended only for healthcare professionals:
Instructions for treatment
Read all the instructions and follow them carefully.
During the procedure described below, aseptic technique should be followed.
The product is reconstituted rapidly at room temperature.
The reconstituted solution should be clear or slightly opalescent. Do not use turbid or sediment-containing solutions. Reconstituted products should be inspected visually for particulate matter and discoloration prior to administration.
Once reconstituted, the solution should be used immediately.
Any unused product or waste material should be disposed of in accordance with local requirements.
Dosage:
Bleeding and prevention of bleeding during treatment with vitamin K antagonists:
The dose will depend on the International Normalized Ratio (INR) prior to treatment and body weight. The following table provides approximate doses (units/kg body weight of the reconstituted product).
INR prior to treatment | 2 – < 4 | 4 – 6 | > 6 |
Dose of Octaplex (units† of factor IX)/kg body weight) | 25 | 35 | 50 |
† Units refer to international units (IU).
The dose is based on body weight up to a maximum of 100 kg. Therefore, for patients weighing more than 100 kg, the maximum single dose (IU of factor IX) should not exceed 2500 IU for an INR of 2 - < 4, 3500 IU for an INR of 4 - 6, and 5000 IU for an INR > 6.
Since these recommendations are empirical and recovery and duration of effect may vary, it is mandatory to monitor INR during treatment.
Bleeding and perioperative prophylaxis in congenital deficiency of coagulation factors II and X when specific purified coagulation factor product is not available:
The calculation of the required dose for treatment is based on the empirical data that approximately 1 IU of factor II or X per kg body weight increases the plasma activity of factor II or X by 0.02 and 0.017 IU/mL, respectively.
Units required = body weight (kg) x desired increase of factor X (IU/mL) x 60
where 60 (mL/kg) is the inverse of the estimated recovery.
Units required = body weight (kg) x desired increase of factor II (IU/mL) x 50
If the individual recovery is known, this value should be used in the calculation.
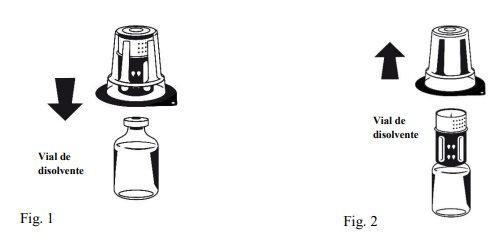
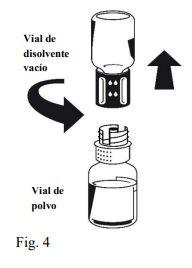
Instructions for reconstitution:
| |
Octaplex dissolves rapidly at room temperature to give a colorless or slightly blueish solution. Unscrew the two parts of the Nextaro® (Fig. 4). Discard the empty solvent vial with the blue part of the Nextaro®. | |
If the concentrate does not dissolve completely or an aggregate forms, the preparation should not be used.
Instructions for perfusion:
As a precautionary measure, the patient's pulse should be measured before and during perfusion. If a marked increase in pulse occurs, the perfusion rate should be reduced or administration should be interrupted.
Once the solution has been transferred, hold the syringe plunger firmly (keeping it facing down) and withdraw the syringe from the Nextaro®. Discard the Nextaro® and the empty vial. |
- Properly disinfect the area where the injection will be applied.
- Inject the solution intravenously at a rate of 0.12 ml/kg/min (≈ 3 units/kg/min), up to a maximum rate of 8 ml/min (≈ 210 units/min), using an aseptic technique.
There should be no blood flow into the syringe due to the risk of fibrin clot formation. The Nextaro® is for single use only.
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS)
http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OCTAPLEX, 500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTIONDosage form: INJECTABLE, 1000 IU/vialActive substance: coagulation factor IX, II, VII and X in combinationManufacturer: Csl Behring GmbhPrescription requiredDosage form: INJECTABLE, 500 IUActive substance: coagulation factor IX, II, VII and X in combinationManufacturer: Csl Behring GmbhPrescription requiredDosage form: INJECTABLE, 250 IUActive substance: coagulation factor IX, II, VII and X in combinationManufacturer: Prothya Biosolutions Netherlands B.V.Prescription required
Online doctors for OCTAPLEX, 500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Discuss questions about OCTAPLEX, 500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions