
NEORECORMON 10000 IU PRE-FILLED SYRINGE SOLUTION FOR INJECTION


How to use NEORECORMON 10000 IU PRE-FILLED SYRINGE SOLUTION FOR INJECTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
NeoRecormon500UI
NeoRecormon2,000UI
NeoRecormon3,000UI
NeoRecormon4,000UI
NeoRecormon5,000UI
NeoRecormon6,000UI
NeoRecormon10,000UI
NeoRecormon20,000UI
NeoRecormon30,000UI
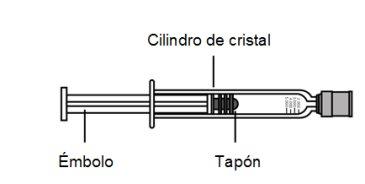
injectable solution in a pre-filled syringe
epoetin beta
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is NeoRecormon and what is it used for
- What you need to know before you use NeoRecormon
- How to use NeoRecormon
- Possible side effects
- Storing NeoRecormon
- Contents of the pack and other information
1. What is NeoRecormon and what is it used for
NeoRecormon is a clear, colourless solution for injection under the skin (subcutaneously) or into a vein (intravenously). It contains epoetin beta, a hormone that stimulates the production of red blood cells. Epoetin beta is produced by specialized genetic technology and works in exactly the same way as the natural human hormone erythropoietin.
You should consult your doctor if you get worse or if you do not improve.
NeoRecormon is indicated for:
- Treatment of symptomatic anaemia caused by chronic kidney disease(renal anaemia) in patients on dialysis or not yet on dialysis.
- Prevention of anaemia in premature babies(with a birth weight of 750 to 1,500 g and a gestational age of less than 34 weeks).
- Treatment of anaemia with symptoms related to cancer in adult patients receiving chemotherapy.
- Treatment of people donating their own blood before surgery.Injections of epoetin beta will increase the amount of blood that can be taken from your body before the operation, to be given to you during or after the operation (this is called autologous transfusion).
2. What you need to know before you use NeoRecormon
Do not useNeoRecormon:
- if you are allergicto epoetin beta or any of the other ingredients of this medicine (listed in section 6).
- if you have uncontrolled high blood pressure
- if you are going to donate your own blood before surgery and:
- you have had a heart attack or stroke in the last month before treatment
- you have unstable angina (new or increasing chest pain)
- you are at risk of blood clots forming in your veins (deep vein thrombosis) - e.g. if you have had clots before.
If you have any of these conditions or could be at risk of them, tell your doctor immediately.
Warnings and precautions
Talk to your doctor before starting NeoRecormon
- If your baby needs treatment with NeoRecormon, your baby will be closely monitoredfor possible effects on the eye
- if your anaemia does not improvewith epoetin treatment
- if you have low levels of certain B vitamins(folic acid or vitamin B12)
- if you have high levels of aluminiumin your blood
- if you have a high platelet count
- if you have chronic liver disease
- if you have epilepsy
- if you have developed antibodies against erythropoietin and pure red cell aplasia(reduced or stopped production of red blood cells) during previous exposure to any erythropoietic substance. In this case, you should not switch to NeoRecormon.
Be careful with other medicines that stimulate red blood cell production:NeoRecormon is one of the agents that stimulate red blood cell production, like the human protein erythropoietin. Your doctor should always record the exact product you are using.
Severe skin reactions, such as Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), have been observed with the administration of epoetins.
SJS/TEN can initially appear as red spots or patches with blisters in the centre, often with a rash on the trunk. Ulcers in the mouth, throat, nose, genitals, and eyes (eye irritation and swelling) may also appear. These severe skin rashes are often preceded by fever and/or flu-like symptoms. The rash may progress to widespread skin peeling and potentially life-threatening complications.
If you experience a severe skin rash or any of these other skin symptoms, stop taking NeoRecormon and contact your doctor or seek medical attention immediately.
Special warning
During treatment with NeoRecormon
If you are a patient with chronic kidney disease,and in particular if you do not respond adequately to NeoRecormon, your doctor will monitor your NeoRecormon dose, as repeated increases in the NeoRecormon dose if you are not responding to treatment may increase the risk of heart or blood vessel problems and may increase the risk of heart attack, stroke, and death.
If you are a cancer patient,you should know that NeoRecormon may act as a growth factor for blood cells and may have a negative effect on the cancer in some circumstances. Depending on your individual situation, a blood transfusion may be preferable. Please discuss this with your doctor.
If you are a patient with kidney disease who has not yet started dialysis,your doctor will decide whether treatment is suitable for you. This is because it cannot be ruled out with certainty that the treatment may accelerate kidney failure.
Your doctor may perform regular blood teststo check:
- your potassium levels. If you have high or increasing potassium levels, your doctor should reconsider your treatment
- your platelet count. During treatment with epoetin, the number of platelets may increase mildly to moderately, and this may lead to changes in blood clotting.
If you are a patient with kidney problems on haemodialysis, your doctor will adjust your heparin dose. This will prevent blockage of the dialysis system tubes.
If you are a patient with kidney problems on haemodialysis and at risk of shunt thrombosis, clots (thrombosis) may form in your shunt (the blood vessel used to connect to the dialysis system). Your doctor may prescribe acetylsalicylic acid or modify the shunt.
If you are donating your own blood before surgery, your doctor will need to:
- check that you can donate blood, especially if your weight is less than 50 kg
- check that you have a sufficient level of red blood cells (haemoglobin of at least 11 g/dl)
- ensure that no more than 12% of your blood volume can be taken at any one time.
Do not misuse NeoRecormon:
Misuse of NeoRecormon by healthy individuals can lead to an increase in blood cells and, as a result, thickening of the blood, which can be associated with life-threatening heart or blood vessel complications.
UsingNeoRecormonwith other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines, including those obtained without a prescription.
Pregnancy, breastfeeding, and fertility
There is limited experience with NeoRecormon in pregnant or breastfeeding women. Ask your doctor or pharmacist for advice before taking any medicine.
NeoRecormon has not shown evidence of altering fertility in animals. The potential risk in humans is unknown.
Driving and using machines
No effects on the ability to drive and use machines have been observed.
NeoRecormon contains phenylalanine and sodium
This medicine contains phenylalanine. It may be harmful to people with phenylketonuria.
If you have phenylketonuria, consult your doctorabout treatment with NeoRecormon.
This medicine contains less than 1 mmol of sodium (23 mg) per dose; this is essentially "sodium-free".
3. How to use NeoRecormon
Always use this medicine exactly as your doctor has told you. If you are not sure, consult your doctor or pharmacist.
Your doctor will use the lowest effective dose to control the symptoms of your anaemia.
If you do not respond adequately to NeoRecormon, your doctor will check your dose and inform you if you need to change the doses.
Treatment should be started under the supervision of a doctor.
Other injections will be given by your doctor or, after being trained, you may inject NeoRecormon yourself (see instructions at the end of this leaflet).
NeoRecormon can be injected under the skin in the abdomen, arm, or thigh, or into a vein. Your doctor will decide what is best for you.
Your doctor will perform regular blood tests to check how your anaemia is responding to treatment by measuring your haemoglobin level.
Dose of NeoRecormon
The dose of NeoRecormon depends on the state of your disease, the way the injection is given (under the skin or into a vein), and your body weight. Your doctor will calculate the appropriate dose for you.
Your doctor will use the lowest effective dose to control the symptoms of your anaemia.
If you do not respond adequately to NeoRecormon, your doctor will check your dose and inform you if you need to change the NeoRecormon dose.
- Symptomatic anaemia caused by chronic kidney disease
Injections are given under the skin or into a vein. If the solution is given into a vein, it should be injected over about 2 minutes, e.g. in patients on haemodialysis via the arteriovenous fistula at the end of dialysis.
Patients not on haemodialysis will normally receive injections under the skin.
Treatment with NeoRecormon is divided into two phases:
- Correction of anaemia
The initial dose for injection under the skinis 20 UI per injection per kg of body weight, given three times a week.
After 4 weeks, your doctor will perform tests and may increase your dose to 40 UI/kg per injection, given three times a week. Your doctor may continue to increase your dose at monthly intervals if necessary.
The weekly dose may be divided into daily doses.
The initial dose for injection into a veinis 40 UI per injection per kg of body weight, given three times a week.
After 4 weeks, your doctor will perform tests, and if the response to treatment is not sufficient, may increase your dose to 80 UI/kg per injection, given three times a week. Your doctor may continue to increase the dose at monthly intervals if necessary.
For both types of injections, the maximum dose should not exceed 720 UI per kg of body weight per week.
- Maintenance of red blood cell levels
Maintenance dose: Once your red blood cells have reached an acceptable level, the dose is reduced to half of the dose used to correct anaemia. The weekly dose may be given once a week or divided into three or seven doses per week. If your red blood cells remain stable on a single weekly dose regimen, you may switch to a single dose every two weeks. In this case, a dose increase may be necessary.
Your doctor may adjust your dose every one or two weeksuntil your individual maintenance dose is found.
Childrenwill start treatment following the same guidelines. In clinical trials, children generally required higher doses of NeoRecormon (the younger the child, the higher the dose).
Treatment with NeoRecormon is usually long-term. However, it can be interrupted at any time if necessary.
- Anaemia in premature babies
Injections are given under the skin.
The initial doseis 250 UI per injection per kg of body weight, given three times a week.
It is likely that premature babies who have already received a previous transfusion when starting treatment with NeoRecormon will not benefit as much as those who have not been transfused.
The recommended duration of treatment is 6 weeks.
- Adultswith symptomatic anaemia and cancer treated with chemotherapy
Injections are given under the skin.
Your doctor may start treatment with NeoRecormon if your haemoglobin level is 10 g/dl or less. After starting treatment, your doctor will keep your haemoglobin level between 10 and 12 g/dl.
The initial weekly doseis 30,000 UI. This dose may be given in one weekly injection or divided into 3 to 7 injections per week. Your doctor will take regular blood samples. Based on the test results, your doctor may increase or decrease your dose or stop treatment. Haemoglobin values should not exceed 12 g/dl.
Treatment should continue until 4 weeks after the end of chemotherapy.
The maximum doseshould not exceed 60,000 UI per week.
- Patients donating their own blood before surgery
Injections are given into a vein over 2 minutes or under the skin.
The dose of NeoRecormondepends on your condition, red blood cell levels, and the amount of blood you will donate before surgery.
The dose calculated by your doctor will be given twice a week for 4 weeks. When you donate blood, you will receive NeoRecormon at the end of the donation.
The maximum doseshould not exceed
- for injection into a vein: 1,600 UI per kg of body weight per week
- for injections under the skin: 1,200 UI per kg of body weight per week
If you inject too muchNeoRecormon
Do not increase the dose that your doctor has given you. If you think you have injected more NeoRecormon than you should, contact your doctor. It is unlikely to be serious. Even in the presence of high blood levels, no symptoms of overdose have been observed.
If you forget to useNeoRecormon
If you have missed an injection or injected too little, tell your doctor.
Do not give a double doseto make up for forgotten doses.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
Adverse effects that may affect any patient
- Most patients (very frequent, may affect more than 1 in 10 people) have low iron levels in the blood. Almost all patients must be treated with iron supplements during treatment with NeoRecormon.
- Rarely (may affect up to 1 in 1,000 people), allergic reactions or skin reactionssuch as rash or hives or reactions at the injection site have appeared.
- Very rarely (may affect up to 1 in 10,000 people), severe forms of allergic reactionhave appeared, especially after injection. These must be treated immediately. If you experience wheezing or difficulty breathing; swelling of the tongue, face, throat, or around the injection site; if you feel dizzy or faint or if you fall, call your doctor immediately.
- Very rarely (may affect up to 1 in 10,000 people), people experience flu-like symptoms, especially when starting treatment. These symptoms includefever, chills, headaches, limb pain, bone pain, and/or general malaise. These reactions were usually mild or moderate and disappeared within hours or days.
- Severe skin rashes, such as Stevens-Johnson syndrome and toxic epidermal necrolysis, have been observed with the administration of epoetins. These reactions can appear as red, circular patches or spots on the skin, often with central blisters on the trunk, skin peeling, and ulcers in the mouth, throat, nose, genitals, and eyes, and may be preceded by fever and flu-like symptoms. Stop using Neorecormon if you experience these symptoms and contact your doctor or seek immediate medical attention. See also section 2.
Adverse effects in patients with chronic kidney disease (renal anemia)
- The most common(very frequent, may affect more than 1 in 10 people)adverse effects are increased blood pressure, worsening of existing blood pressure, and headaches. Your doctor should treat high blood pressure with appropriate medications or temporarily interrupt treatment with NeoRecormon.
- If you experience headaches, especially sudden, sharp, migraine-like headaches, confusion, speech disorders, instability while walking, seizures, or convulsions, call your doctor immediately. These may be symptoms of extremely high blood pressure (hypertensive crisis), even if your blood pressure is normally normal or low. This symptomatology must be treated immediately.
- If you have low blood pressure or dialysis complications, you may experience a dialysis shunt thrombosis(a blood clot in the vessel used to connect to the dialysis system).
- Very rarely (may affect up to 1 in 10,000 people), patients have high levels of potassium or phosphatesin their blood. These can be treated by your doctor.
- Pure red cell aplasia (PRCA) caused by neutralizing antibodies has been observed during treatment with erythropoietin, including some isolated cases during treatment with NeoRecormon. PRCA means that the body stops or reduces the production of red blood cells. This causes severe anemia, whose symptoms include unusual fatigue and lack of energy. If your body produces neutralizing antibodies, your doctor will interrupt therapy with NeoRecormon and determine the best course of action to treat your anemia.
Additional adverse effects in adults with cancer treated with chemotherapy
- Occasionally, an increase in blood pressure and headachesmay occur. Your doctor may treat high blood pressure with medications.
- An increase in the incidence of blood clotshas been observed.
Additional adverse effects in patients who donate their own blood before undergoing surgery
- A slight increase in the incidence of blood clotshas been observed.
Reporting of adverse effects
If you experience any type of adverse effect, consult your doctor or nurse, even if it is a possible adverse effect that is not listed in this prospectus. You can also report them directly through the national reporting system included in Appendix V. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of NeoRecormon
- Keep this medicine out of the sight and reach of children.
- Do not use NeoRecormon after the expiration date stated on the carton and label.
- Store in a refrigerator (between 2°C and 8°C).
- The syringe can be stored outside the refrigerator for a single period of up to 3 days at room temperature (not above 25°C).
- Keep the pre-filled syringe in the outer packaging to protect it from light.
- Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and any unused medicines. This will help protect the environment.
6. Container Contents and Additional Information
Composition of NeoRecormon
- The active ingredient is epoetin beta. 1 pre-filled syringe contains 500, 2,000, 3,000, 4,000, 5,000, 6,000, 10,000, 20,000 or 30,000 IU (International Units) of epoetin beta in 0.3 ml or 0.6 ml of solution.
- The other components are:
urea, sodium chloride, polysorbate 20, monosodium phosphate dihydrate, disodium phosphate dodecahydrate, calcium chloride dihydrate, glycine, L-Leucine, L-Isoleucine, L-Threonine, L-Glutamic acid, and L-Phenylalanine, and water for injectable preparations. (See section 2 "NeoRecormon contains phenylalanine and sodium").
Appearance of NeoRecormon and Container Contents
NeoRecormon is a solution in a pre-filled syringe for injection.
The Solution is colorless, from transparent to slightly opalescent.
NeoRecormon 500 IU, 2,000 IU, 3,000 IU, 4,000 IU, 5,000 IU, and 6,000 IU: each pre-filled syringe contains 0.3 ml of solution.
NeoRecormon 10,000 IU, 20,000 IU, and 30,000 IU: each pre-filled syringe contains 0.6 ml of solution.
NeoRecormon is presented in the following pack sizes:
NeoRecormon 500 IU
1 pre-filled syringe with 1 needle (30G1/2) or
6 pre-filled syringes with 6 needles (30G1/2).
NeoRecormon 2,000 IU, 3,000 IU, 4,000 IU, 5,000 IU, 6,000 IU, 10,000 IU, and 20,000 IU
1 pre-filled syringe with 1 needle (27G1/2) or
6 pre-filled syringes with 6 needles (27G1/2).
NeoRecormon 30,000 IU
1 pre-filled syringe with 1 needle (27G1/2) or
4 pre-filled syringes with 4 needles (27G1/2).
Not all pack sizes may be marketed.
Marketing Authorisation Holder
Roche Registration GmbH
Emil-Barell-Strasse 1
79639 Grenzach-Wyhlen
Germany
Manufacturer
Roche Pharma AG
Emil-Barell-Strasse 1
D-79639 Grenzach-Wyhlen
Germany
You can request more information about this medicinal product from the local representative of the marketing authorisation holder:
|
Czech Republic Roche s.r.o. Tel.: +420 - 2 20382111 | Hungary Roche (Hungary) Kft. Tel.: +36 - 1 279 4500 |
Denmark Roche Pharmaceuticals A/S Tel.: +45 - 36 39 99 99 | Malta (See Ireland) |
Germany Roche Pharma AG Tel.: +49 (0) 7624 140 | Netherlands Roche Nederland B.V. Tel.: +31 (0) 348 438050 |
Estonia Roche Eesti OÜ Tel.: + 372 - 6 177 380 | Norway Roche Norge AS Tel.: +47 - 22 78 90 00 |
Greece Roche (Hellas) A.E. Tel.: +30 210 61 66 100 | Austria Roche Austria GmbH Tel.: +43 (0) 1 27739 |
Spain Roche Farma S.A. Tel.: +34 - 91 324 81 00 | Poland Roche Polska Sp.z o.o. Tel.: +48 - 22 345 18 88 |
France Roche Tel: +33 (0) 1 47 61 40 00 | Portugal Roche Farmacêutica Química, Lda Tel.: +351 - 21 425 70 00 |
Croatia Roche d.o.o. Tel.: +385 1 4722 333 | Romania Roche România S.R.L. Tel.: +40 21 206 47 01 |
Ireland Roche Products (Ireland) Ltd. Tel.: +353 (0) 1 469 0700 | Slovenia Roche farmacevtska družba d.o.o. Tel.: +386 - 1 360 26 00 |
Iceland Roche Pharmaceuticals A/S c/o Icepharma hf Tel.: +354 540 8000 | Slovakia Roche Slovensko, s.r.o. Tel.: +421 - 2 52638201 |
Italy Roche S.p.A. Tel.: +39 - 039 2471 | Finland Roche Oy Tel: +358 (0) 10 554 500 |
Cyprus Γ.Α.Σταμ?της & Σια Λτδ. Tel.: +357 - 22 76 62 76 | Sweden Roche AB Tel.: +46 (0) 8 726 1200 |
Latvia Roche Latvija SIA Tel.: +371 - 6 7039831 | United Kingdom (Northern Ireland) Roche Products (Ireland) Ltd. Tel: +44 (0) 1707 366000 |
Date of Last Revision of this Leaflet
Detailed information on this medicinal product is available on the European Medicines Agency website http://www.ema.europa.eu/
NeoRecormon Pre-filled Syringe
Instructions for Use
The following instructions explain how to administer an injection of NeoRecormon. Make sure to read, understand, and follow the instructions for use as well as the leaflet before injecting NeoRecormon. Your healthcare professional will show you how to prepare and inject NeoRecormon correctly before you use it for the first time.
Do notinject yourself unless you have received training. Consult your healthcare professional if you need further training.
Always follow all the instructions in these instructions for use, as they may differ from your experiences. These instructions will minimize risks such as accidental needlestick and prevent incorrect use.
NeoRecormon can be administered in 2 ways, your doctor will decide which way is suitable for you:
- Intravenous administration (in the vein or venous access), only to be performed by healthcare professionals.
- Subcutaneous administration (under the skin).
Before Starting to Use
- Do not remove the needle cap until you are ready to inject NeoRecormon.
- Do not attempt to remove the syringe at any time.
- Do not reuse the same syringe.
- Do not use it if the syringe has been dropped or is damaged.
- Do not leave the syringe unattended.
- Keep the syringe and needle, as well as the puncture-proof container or sharp object container, out of the reach of children.
- If you have any questions, contact your healthcare professional.
Storage Instructions
- Store the unused syringe(s) and needles in the original packaging and keep them in the refrigerator between 2 °C and 8 °C.
- Keep the syringe and needle away from direct sunlight.
- Do not freeze.
- Do not use if the syringe has been frozen.
- Always keep the syringe and needle dry.
Materials Needed to Administer the Injection
Included in the Packaging:
- NeoRecormon pre-filled syringe(s).
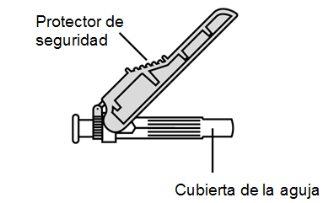
- Injection needle(s) (27G or 30G) (depending on the prescribed dose of the medication) with safety protector (used for preparation, dose adjustment, and injection of the medication).
Note: Each NeoRecormon packaging contains 1 syringe/1 needle, 4 syringes/4 needles, or 6 syringes/6 needles.
- Instructions for use and leaflet.
Not Included in the Packaging:
- 1 alcohol swab.
- 1 dry sterile gauze.
- 1 puncture-proof container or sharp object container for the safe disposal of the rubber cap, needle cap, and used syringe.
Preparation for Injection
- Find a well-lit, clean, and flat work surface.
- Take the carton with the syringe(s) and needle(s) out of the refrigerator
- Check the packaging, the perforations on the front, and the seal. Also, check the expiration date.
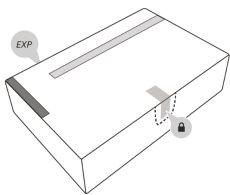
- Do notuse if the expiration date has passed, or if the packaging appears to be tampered with. In this case, proceed to step 20and contact your healthcare professional.
- Do notuse if the perforations or seal are broken. In this case, proceed to step 20and contact your healthcare professional.
- Open the packaging by pushing through the perforation around the seal.
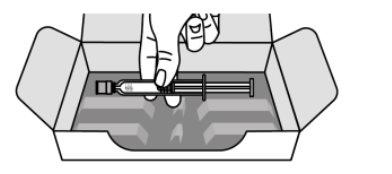
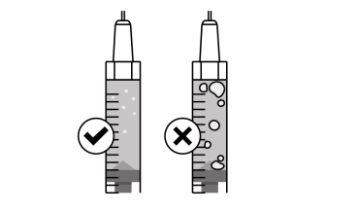
- Take a syringe out of the carton and a needle from the needle box. Be careful when removing the syringe. Make sure to always hold the syringe as shown in the image below.
- Do notturn the carton upside down to remove the syringe.
- Do nothandle the syringe by holding the plunger or the needle cap.
Observation:If you have a multipack, put the carton with the remaining syringe(s) and needle(s) back in the refrigerator.
- Inspect the syringe and needle closely
- Check that the syringe and needle are not damaged. Do notuse the syringe if it has been dropped or if any part of the syringe appears to be damaged.
- Check the expiration date on the syringe and needle. Do notuse the syringe or needle if the expiration date has passed.
- Check the liquid in the syringe. The liquid should be transparent and colorless. Do notuse the syringe if the liquid is cloudy, discolored, or has particles.
- Place the syringe on a clean and flat surface.
- Wash your hands with water and soap.
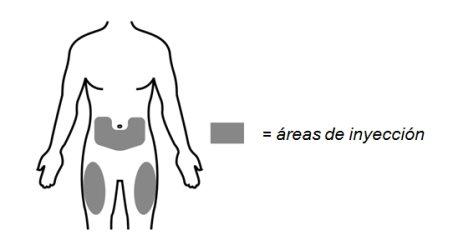
- Choose an injection site:
- The recommended injection sites are the top of your thigh or the lower part of your abdomen below the navel. Do notinject within the area of 5 cm (2 inches) directly around your navel.
- Choose a different injection site for each new injection.
- Do notinject into moles, scars, bruises, or areas where the skin is sensitive, red, hard, or not intact.
- Do notinject into a vein or muscle.
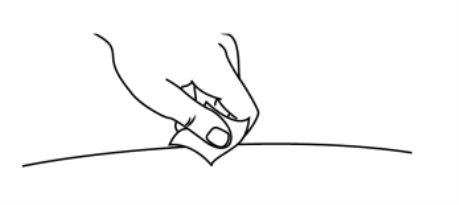
- Clean the injection site with an alcohol swab and let it air dry for 10 seconds.
- Do notfan or blow on the cleaned area.
- Do nottouch the injection site again before administering the injection.
Subcutaneous Injection Administration
- Slide the safety protector back towards the syringe barrel.
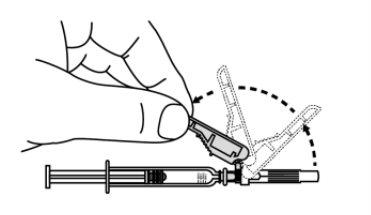
- Hold the syringe and needle firmly in the axis and carefully remove the needle cap from the syringe. Use the syringe within 5 minutes after removing the cap; otherwise, the needle may become clogged.
- Do nothold the plunger while removing the needle cap.
- Do nottouch the needle after removing the needle cap.
- Do notrecap the needle.
- Do notstraighten the needle if it is bent or damaged.
Discard the needle cap in the puncture-proof container immediately.
- Hold the syringe with the needle facing upwards. Remove any large air bubbles by gently tapping the syringe barrel with your fingers until the air bubbles rise to the top of the syringe. Then, slowly push the plunger upwards to push the air bubbles out of the syringe.
- Adjust the prescribed dose by slowly pushing the plunger.
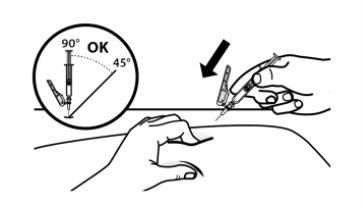
- Pinch the selected injection site and insert the needle completely at a 45° to 90° angle with a quick and firm action.
- Do nottouch the plunger while inserting the needle into the skin
- Do notinsert the needle through clothing.
Once you have inserted the needle, release the pinch and hold the syringe firmly in place.
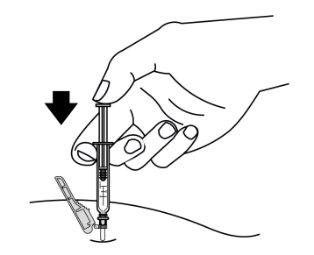
- Inject the prescribed dose slowly by gently pushing the plunger until it reaches the bottom
- Remove the needle and syringe from the injection site at the same angle as you inserted it.
After the Injection
- There may be some bleeding at the injection site. You can press a dry sterile gauze over the injection site. Do notrub the injection site.
- If necessary, you can cover the injection site with a small bandage.
- In case of contact with the medication, wash the area that came into contact with the medication with water.
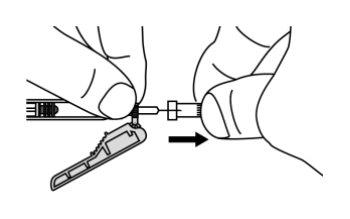
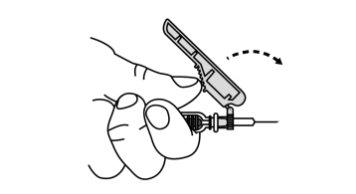
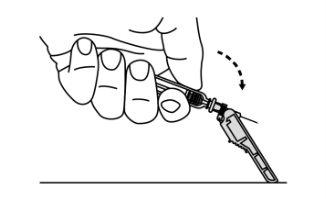
- Slide the safety protector forward 90°, away from the syringe barrel (A).
Holding the syringe with one hand, press the safety protector against a flat surface with a firm and quick motion until you hear a "click" (B).
- If you do not hear a click, check that the needle is fully covered by the safety protector.
- Keep your fingers behind the safety protector and away from the needle at all times.
A)
B)
- Put the used syringe in a puncture-proof container immediately after use.
- Do notattempt to remove the used injection needle from the used syringe.
- Do notrecap the injection needle.
- Do notthrow the syringe in your household trash.
Important:Always keep the puncture-proof container out of the reach of children.
<---------------------------------------------------------------------------------------------------------------------->
Instructions for Use for Intravenous Injection, Intended Only for Healthcare Professionals
The following instructions for use explain how to administer an intravenous injection of NeoRecormon. Make sure to read, understand, and follow the instructions for use, as well as the leaflet, before injecting NeoRecormon.
Intravenous Injection Administration (only for healthcare professionals).
Preparation for injection: follow steps 1 to 9 of subcutaneous injection (above).
- Select a vein. Change veins for each injection to prevent pain at one site.
- Do notinject into a red or swollen area.
- Do notinject into a muscle.
Clean the skin over the vein with an alcohol swab and let it air dry.
- Do notfan or blow on the cleaned area.
- Do nottouch the injection site again before administering the injection.
- Prepare the syringe and needle: follow steps 12 to 15 of subcutaneous injection (above).
- Insert the needle into the vein.
- Do nothold or push the plunger while inserting the needle.
- Inject the prescribed dose slowly by gently pushing the plunger until it reaches the bottom. Remove the needle and syringe from the injection site at the same angle as you inserted it.
After the injection, follow steps 18 to 20 of subcutaneous injection (above)
Administer the intravenous injection through the injection port (for healthcare professionals only).Preparation for injection: follow steps 1 to 9 of subcutaneous injection (above).
- Clean the skin around the injection site with an alcohol swab and let it dry.
Clean the injection site according to the device provider's instructions.
- Do notventilate or blow on the cleaned area.
- Do nottouch the injection site again before administering the injection.
- Prepare the syringe and needle: follow steps 12 to 15 of subcutaneous injection (above)
- Insert the needle into the injection site (follow the instructions of the venous access device provider)
- Do nothold or push the plunger while inserting the needle.
- Slowly inject the prescribed dose by gently pushing the plunger to the bottom. Remove the needle and syringe from the injection port at the same angle as it was inserted.
After the injection, follow steps 18 to 20 of subcutaneous injection (above).
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NEORECORMON 10000 IU PRE-FILLED SYRINGE SOLUTION FOR INJECTIONDosage form: INJECTABLE, 20000 IUActive substance: erythropoietinManufacturer: Sandoz GmbhPrescription requiredDosage form: INJECTABLE, 20,000 IUActive substance: erythropoietinManufacturer: Sandoz GmbhPrescription requiredDosage form: INJECTABLE, 40,000 IU/ml of epoetin alfaActive substance: erythropoietinManufacturer: Sandoz GmbhPrescription required
Online doctors for NEORECORMON 10000 IU PRE-FILLED SYRINGE SOLUTION FOR INJECTION
Discuss questions about NEORECORMON 10000 IU PRE-FILLED SYRINGE SOLUTION FOR INJECTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions
















