
ИБУПРОФЕН СТАДАФАРМ 400 МГ ОРАЛЬНАЯ СУСПЕНЗИЯ

Спросите врача о рецепте на ИБУПРОФЕН СТАДАФАРМ 400 МГ ОРАЛЬНАЯ СУСПЕНЗИЯ

Инструкция по применению ИБУПРОФЕН СТАДАФАРМ 400 МГ ОРАЛЬНАЯ СУСПЕНЗИЯ
Введение
Инструкция: информация для пользователя
Ибупрофен Стадафарм 400 мг суспензия для приема внутрь
Прочитайте внимательно всю инструкцию перед началом приема этого лекарства, поскольку она содержит важную информацию для вас.
Следуйте точно инструкциям по применению лекарства, содержащимся в этой инструкции или указанным вашим врачом или фармацевтом.
- Сохраните эту инструкцию, поскольку вам может потребоваться прочитать ее снова.
- Если вам нужен совет или дополнительная информация, проконсультируйтесь с вашим фармацевтом.
- Если вы испытываете побочные эффекты, проконсультируйтесь с вашим врачом или фармацевтом, даже если это побочные эффекты, которые не указаны в этой инструкции. См. раздел 4.
- Вам следует проконсультироваться с врачом, если температура тела повышается или не улучшается после 3 дней или боль после 5 дней (3 дня у подростков).
Содержание инструкции
- Что такое Ибупрофен Стадафарм и для чего он используется
- Что вам нужно знать перед началом приема Ибупрофена Стадафарма
- Как принимать Ибупрофен Стадафарм
- Возможные побочные эффекты
- Хранение Ибупрофена Стадафарма
- Содержание упаковки и дополнительная информация
1. Что такое Ибупрофен Стадафарм и для чего он используется
Ибупрофен, активное вещество этого лекарства, действует путем уменьшения боли и температуры.
Это лекарство используется у взрослых и подростков старше 12 лет и с весом более 40 кг для симптоматического облегчения легких или умеренных болей, таких как головные боли, зубные боли, менструальные боли, мышечные боли (спазмы) или боли в спине (люмбаго), а также при лихорадочных состояниях.
2. Что вам нужно знать перед началом приема Ибупрофена Стадафарма
Не принимайте Ибупрофен Стадафарм:
- Если вы аллергичны к ибупрофену или любому другому компоненту этого лекарства (указанному в разделе 6) или к другим лекарствам группы нестероидных противовоспалительных препаратов (НПВП) или к аспирину. Реакции, указывающие на аллергию, могут быть: кожная сыпь с зудом, отек лица, губ или языка, насморк, затруднение дыхания или астма.
- Если у вас ранее была язва или кровотечение желудка или двенадцатиперстной кишки или вы перенесли перфорацию желудочно-кишечного тракта.
- Если вы рвете кровью.
- Если у вас есть черные калы или диарея с кровью.
- Если у вас тяжелая сердечная недостаточность.
- Если у вас тяжелое заболевание печени или почек.
- Если у вас есть кровотечения или нарушения свертываемости крови, или вы принимаете антикоагулянты (лекарства, используемые для «разжижения» крови). Если необходимо одновременно принимать антикоагулянты, врач проведет тесты на свертываемость крови.
- Если вы находитесь на третьем триместре беременности.
- Если у вас тяжелая дегидратация (вызванная рвотой, диареей или недостаточным потреблением жидкости).
Предостережения и меры предосторожности
Проконсультируйтесь с вашим врачом или фармацевтом перед началом приема этого лекарства.
Сообщите вашему врачу:
- Если у вас есть отеки (задержка жидкости)
- Если у вас есть или были заболевания сердца или высокое кровяное давление.
- Если у вас астма или любое другое респираторное заболевание.
- Если вы получаете лечение этим лекарством, поскольку оно может маскировать температуру, которая является важным признаком инфекции, затрудняя ее диагностику.
- Если у вас есть заболевание почек или печени, вам больше 60 лет или вам необходимо принимать лекарство в течение длительного периода (более 1-2 недель), возможно, ваш врач будет проводить регулярные проверки. Ваш врач укажет частоту этих проверок.
- Если у вас出现ят симптомы дегидратации, например, сильная диарея или рвота, принимайте много жидкости и немедленно свяжитесь с вашим врачом, поскольку ибупрофен в этом случае может вызвать почечную недостаточность в результате дегидратации.
- Если у вас была или развилась язва, кровотечение или перфорация желудка или двенадцатиперстной кишки, которая может проявляться сильной болью в животе или постоянной болью и/или черными калами, или даже без предшествующих симптомов. Этот риск выше, когда используются высокие дозы и длительное лечение, у пациентов с язвенной болезнью и у пожилых пациентов. В этих случаях ваш врач рассмотрит возможность сочетания с лекарством, защищающим желудок.
- Если вы одновременно принимаете лекарства, которые нарушают свертываемость крови, такие как антикоагулянты, также необходимо обсудить использование других лекарств, которые могут увеличить риск таких кровотечений, как кортикостероиды и антидепрессанты.
- Если у вас болезнь Крона (хроническое заболевание, при котором иммунная система атакует кишечник, вызывая воспаление, которое обычно приводит к диарее с кровью) или язвенный колит, поскольку лекарства типа ибупрофена могут ухудшать эти заболевания.
- Если вы проходите лечение диуретиками (лекарствами для мочеиспускания), поскольку ваш врач должен контролировать функцию почек.
- Если у вас системный красный люпус (хроническое заболевание, которое влияет на иммунную систему и может поражать различные жизненно важные органы, нервную систему, кровеносные сосуды, кожу и суставы), поскольку может произойти асептический менингит.
- Если у вас межмитотическая порфирия (метаболическое заболевание, которое влияет на кровь и может вызывать симптомы, такие как красноватая окраска мочи, кровь в моче или заболевания печени), чтобы оценить целесообразность или нет лечения ибупрофеном.
- Если вы испытываете головные боли после длительного лечения, не принимайте более высокие дозы лекарства.
- Возможно, что могут возникнуть аллергические реакции на это лекарство.
- Врач будет проводить более строгий контроль, если вы принимаете ибупрофен после прохождения крупной операции.
- Рекомендуется не принимать это лекарство, если у вас ветрянка.
- Если у вас инфекция; см. раздел «Инфекции» ниже.
- С ибупрофеном были зарегистрированы признаки аллергической реакции на это лекарство, такие как респираторные проблемы, отек лица и шеи (ангиоэдема) и боль в груди. Прекратите использовать это лекарство немедленно и обратитесь за медицинской помощью, если вы заметите любой из этих признаков.
Важно использовать самую маленькую дозу, которая облегчает/контролирует боль, и не принимать это лекарство дольше, чем необходимо для контроля симптомов.
Инфекции
Ибупрофен Стадафарм может маскировать признаки инфекции, такие как температура и боль. Следовательно, возможно, что Ибупрофен Стадафарм задержит адекватное лечение инфекции, что может увеличить риск осложнений. Это было замечено при пневмонии, вызванной бактериями, и при бактериальных инфекциях кожи, связанных с ветрянкой. Если вы принимаете это лекарство во время инфекции и симптомы инфекции сохраняются или ухудшаются, проконсультируйтесь с врачом без задержки.
Предостережения при сердечно-сосудистых заболеваниях
Противовоспалительные/обезболивающие лекарства, такие как ибупрофен, могут быть связаны с небольшим увеличением риска сердечного приступа или инсульта, особенно при использовании в высоких дозах. Не превышайте рекомендуемую дозу или продолжительность лечения. Вам следует обсудить лечение с вашим врачом или фармацевтом перед приемом этого лекарства, если:
- у вас есть проблемы с сердцем, включая сердечную недостаточность, ангину (боль в груди) или если вы перенесли сердечный приступ, операцию на коронарных артериях, периферическую артериальную болезнь (проблемы с кровообращением в ногах или ступнях из-за сужения или блокировки артерий) или любой тип инсульта (включая «мини-инсульт» или транзиторную ишемическую атаку).
- у вас высокое кровяное давление, диабет, высокий уровень холестерина, семейная история сердечно-сосудистых заболеваний или инсульта, или если вы курите.
Кроме того, этот тип лекарств может вызывать задержку жидкости, особенно у пациентов с сердечной недостаточностью и/или высоким кровяным давлением (гипертонией).
Кожные реакции
Были зарегистрированы тяжелые кожные реакции, такие как эксфолиативная дерматит, эритема мультиформе, синдром Стивенса-Джонсона, токсический эпидермальный некролиз, реакция на лекарство с эозинофилией и системными симптомами (синдром DRESS), острый генерализованный пустулезный экзантем, в связи с лечением ибупрофеном. Прекратите лечение Ибупрофеном Стадафармом и немедленно обратитесь за медицинской помощью, если вы заметите любой из этих симптомов, связанных с тяжелыми кожными реакциями, описанных в разделе 4.
Дети и подростки
Существует риск повреждения почек у детей и подростков, страдающих дегидратацией.
Прием Ибупрофена Стадафарма с другими лекарствами
Сообщите вашему врачу или фармацевту, если вы принимаете, недавно принимали или можете принимать любое другое лекарство, включая те, которые можно купить без рецепта.
Лекарства, перечисленные ниже, могут взаимодействовать и, следовательно, не должны приниматься вместе с ибупрофеном без предварительной консультации с врачом:
- Не принимайте это лекарство, если вы принимаете другие нестероидные противовоспалительные препараты (НПВП), такие как аспирин, поскольку это может увеличить риск язвы и кровотечения в желудочно-кишечном тракте.
- Антиагреганты (препятствующие образованию тромбов или сгустков в кровеносных сосудах), такие как тиклопидин.
- Антикоагулянты (например, для лечения проблем со свертываемостью/предотвращения свертывания, например, ацетилсалициловая кислота, варфарин).
- Лекарства, снижающие высокое кровяное давление (ингибиторы АПФ, такие как каптоприл, бета-блокаторы, такие как атенолол, и антагонисты рецепторов ангиотензина II, такие как лозартан).
- Баклофен (используемый для лечения непроизвольных мышечных сокращений).
- Литий (лекарство, используемое для лечения депрессии). Возможно, ваш врач отрегулирует дозу этого лекарства.
- Метотрексат (для лечения рака и воспалительных заболеваний). Возможно, ваш врач отрегулирует дозу этого лекарства.
- Мифепристон (индуктор абортов).
- Дигоксин (сердечные гликозиды) (используемые для лечения сердечных расстройств).
- Гидантоины, такие как фенитоин (используемые для лечения эпилепсии).
- Сульфамиды, такие как сульфаметоксазол и ко-тримоксазол (используемые для лечения некоторых бактериальных инфекций).
- Диуретики (лекарства, используемые для увеличения выведения мочи).
- Кортикостероиды, такие как кортизон и преднизолон, используемые при воспалительных процессах.
- Селективные ингибиторы обратного захвата серотонина (СИОЗС), используемые при депрессии.
- Пентоксифиллин (для лечения интермиттирующей хромоты).
- Пробенецид (используемый у пациентов с подагрой или в сочетании с пенициллином при инфекциях).
- Антибиотики группы хинолонов, такие как норфлоксацин.
- Ионообменные смолы, такие как холестирамин (используемый для снижения уровня холестерина в крови).
- Сульфинпиразон (для подагры).
- Сульфонилмочевины, такие как толбутамид (для диабета).
- Такрин (используемый для лечения болезни Альцгеймера).
- Такролимус или циклоспорин (используемые при трансплантации органов для предотвращения отторжения).
- Зидовудин (лекарство против вируса СПИДа).
- Тромболитические препараты (лекарства, растворяющие тромбы).
- Антибиотики аминогликозиды, такие как нейомицин.
- Ингибиторы CYP2C9 (ответственные за метаболизм многих лекарств в печени), такие как вориконазол или флуконазол, используемые для лечения грибковых инфекций.
- Травяные экстракты: экстракт Гинкго билоба.
Другие лекарства также могут влиять или быть затронуты лечением ибупрофеном. Поэтому вы должны всегда консультироваться с вашим врачом или фармацевтом перед использованием ибупрофена с другими лекарствами.
Взаимодействие с аналитическими тестами
Если вам предстоит пройти какие-либо диагностические тесты (включая анализы крови, мочи, кожные пробы с аллергенами и т. д.), сообщите врачу, что вы принимаете это лекарство, поскольку оно может изменить результаты.
Прием Ибупрофена Стадафарма с пищей, напитками и алкоголем
Вы можете принимать его один или с пищей. Обычно рекомендуется принимать его перед едой, чтобы уменьшить вероятность появления неприятных ощущений в желудке.
Если вы принимаете алкоголь во время лечения этим лекарством, вы можете быть более склонны к побочным эффектам.
Беременность, лактация и фертильность
Если вы беременны или кормите грудью, считаете, что можете быть беременной или планируете стать беременной, проконсультируйтесь с вашим врачом или фармацевтом перед использованием этого лекарства.
Беременность
Не принимайте ибупрофен, если вы находитесь в последних 3 месяцах беременности, поскольку это может нанести вред плоду или вызвать проблемы во время родов. Это может вызвать проблемы с почками и сердцем у вашего ребенка. Это может повлиять на вашу и вашего ребенка способность к свертываемости крови и задержать или продлить роды больше, чем ожидается. Не принимайте ибупрофен в течение первых 6 месяцев беременности, если это не абсолютно необходимо и если ваш врач не указал иного. Если вам необходимо лечение в этот период или во время попыток зачатия, вам следует принимать минимальную дозу в течение самого короткого возможного времени. После 20-й недели беременности ибупрофен может вызвать проблемы с почками у вашего ребенка, если принимать его более нескольких дней, что может привести к низкому уровню околоплодных вод, окружающих ребенка (олигогидрамнию), или сужению кровеносного сосуда (ductus arteriosus) в сердце ребенка. Если вам необходимо лечение в течение более длительного периода, ваш врач может рекомендовать дополнительные проверки.
Фертильность
Для пациенток детородного возраста следует учитывать, что лекарства, такие как ибупрофен, были связаны с уменьшением способности к зачатию.
Лактация
Хотя уровни лекарства в грудном молоке не обнаруживаются, рекомендуется проконсультироваться с врачом при длительном лечении или приема высоких доз во время лактации.
Вождение и использование машин
Если вы испытываете головокружение, вертиго, нарушения зрения или другие симптомы, принимая это лекарство, не следует водить транспорт или использовать машины. Если вы принимаете только одну дозу ибупрофена или в течение короткого периода, не необходимо принимать специальных мер предосторожности.
Ибупрофен Стадафарм содержит натрий и жидкий мальтитол (Е-965)
Пациенты с диетами, бедными натрием, должны учитывать, что это лекарство содержит 57,94 мг (2,5 ммоль) натрия на каждый пакет.
Это лекарство содержит жидкий мальтитол (Е-965). Если ваш врач указал, что у вас непереносимость некоторых сахаров, проконсультируйтесь с ним перед приемом этого лекарства.
3. Как принимать Ибупрофен Стадафарм
Следуйте точно инструкциям по применению препарата, указанным в этом листке-вкладыше и рекомендованным вашим врачом или фармацевтом. В случае сомнений обратитесь к врачу или фармацевту.
Важно всегда принимать самую маленькую дозу, которая облегчает боль, и не принимать препарат дольше, чем необходимо для контроля симптомов.
Следует использовать эффективную дозу в течение минимального времени, необходимого для облегчения симптомов. Если у вас есть инфекция, обратитесь к врачу без промедления, если симптомы (такие как температура и боль) сохраняются или ухудшаются (см. раздел 2).
Посология
Взрослые и подростки (с весом более 40 кг) от 12 лет:
Рекомендуемая доза составляет один пакет (400 мг ибупрофена) каждые 4-8 часов, в зависимости от интенсивности симптомов и реакции на лечение. Не принимайте более 3 пакетов (1200 мг) в течение 24 часов.
Пациенты пожилого возраста:
Дозировку должен устанавливать врач, поскольку может потребоваться снижение обычной дозы. Пожилые люди чаще испытывают побочные эффекты.
Пациенты с заболеваниями почек, печени или сердца:
Снизьте дозу и проконсультируйтесь с врачом. Ибупрофен не должен применяться у пациентов с тяжелой почечной, печеночной или сердечной недостаточностью.
Если симптомы ухудшаются, если температура сохраняется более 3 дней или боль более 5 дней (3 дня у подростков), обратитесь к врачу.
Применение этого препарата зависит от появления боли или температуры. По мере исчезновения этих симптомов следует прекратить прием препарата.
Применение у детей и подростков:
Не рекомендуется применение этого препарата у детей и подростков с весом менее 40 кг.
Форма применения
Этот препарат представляет собой суспензию, которая принимается перорально.
Необходимо перемешать суспензию перед приемом, как показано на следующей иллюстрации:
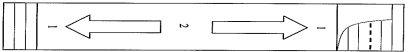
1 – Нажмите пальцами на верхнюю и нижнюю часть пакета несколько раз
2 – Нажмите на верхнюю и нижнюю части и наоборот в течение минимум 30 секунд
Препарат можно принимать непосредственно из пакета или разбавленным в воде.
В случае желудочно-кишечных расстройств рекомендуется принимать препарат с пищей.
Если вы приняли больше Ибупрофена Стадафарма, чем следует
Если вы приняли больше суспензии, чем следует, или случайно проглотили содержимое упаковки, обратитесь немедленно к врачу или фармацевту или в Центр токсикологической информации, телефон: 91 5620420, указав препарат и количество, принятое внутрь. Рекомендуется взять с собой упаковку и листок-вкладыш препарата к медицинскому специалисту.
Если вы приняли больше Ибупрофена Стадафарма, чем следует, или если ребенок случайно проглотил препарат, обратитесь к врачу или в ближайшую больницу, чтобы узнать о риске и получить советы о мерах, которые необходимо принять.
Симптомы передозировки могут включать тошноту, боль в желудке, рвоту (которая может содержать кровавый мокрот), головную боль, звон в ушах, путаницу и непроизвольные движения глаз. При высоких дозах были зарегистрированы симптомы сонливости, боли в груди, сердцебиения, потери сознания, судорог (в основном у детей), слабости и головокружения, крови в моче, низкого уровня калия в крови, озноба и проблем с дыханием
Редко могут возникать более тяжелые симптомы, такие как желудочно-кишечное кровотечение, снижение артериального давления, снижение температуры тела, метаболический ацидоз, судороги, нарушение функции почек, кома, дыхательный дистресс у взрослых и транзиторная остановка дыхания у детей (после приема больших количеств).
Если произошла тяжелая интоксикация, врач примет необходимые меры.
Если вы пропустили прием Ибупрофена Стадафарма
Не принимайте двойную дозу, чтобы компенсировать пропущенные дозы.
Если вы забыли принять свою дозу, примите ее как можно скорее. Однако, если время следующего приема уже близко, пропустите пропущенную дозу и примите следующую дозу в обычное время.
Если у вас есть какие-либо другие вопросы о применении этого препарата, обратитесь к врачу или фармацевту.
4. Возможные побочные эффекты
Как и все лекарства, этот препарат может вызывать побочные эффекты, хотя не все люди испытывают их.
Частота побочных эффектов меньше при коротком лечении и если суточная доза ниже максимальной рекомендуемой дозы.
Частоты, указанные ниже, относятся к краткосрочному применению максимальных суточных доз до 1200 мг ибупрофена перорально:
- Частые побочные эффекты(могут возникать у до 1 из 10 человек): желудочно-кишечные кровотечения, особенно у пациентов пожилого возраста. Также были зарегистрированы тошнота, рвота, диарея, метеоризм, диспепсия (нарушение секреторной или двигательной функции желудочно-кишечного тракта), запор, изжога, боль в животе, кровь в кале, рвота с кровью, головокружение или чувство неустойчивости, усталость.
- Редкие побочные эффекты(могут возникать у до 1 из 100 человек): были зарегистрированы гастрит, язвы двенадцатиперстной кишки, язвы желудка, покраснение кожи, зуд или онемение кожи, крапивница, пурпура (фиолетовые пятна на коже), реакции на кожу под влиянием света, гиперчувствительность, парестезия (чувство онемения, покалывания, жжения и т. д., чаще в руках, ногах, руках или ногах), головная боль и сонливость, бессонница, тревога, нарушения слуха, нарушения зрения, ринит (воспаление слизистой оболочки носа), воспаление слизистой оболочки рта с образованием язв (афты), желудочно-кишечные перфорации, гепатит (воспаление печени), аномалии функции печени и желтуха (желтый цвет кожи и глаз), астма, бронхоспазм (затруднение дыхания).
- Очень редкие побочные эффекты(могут возникать у до 1 из 1000 человек):
Дезориентация или путаница, депрессия, вертиго, звон в ушах, тиннитус, амблиопия, токсическая обратимая, повреждения печени, отек (отек, вызванный накоплением жидкости в тканях), неврит зрительного нерва.
- Очень редкие побочные эффекты(могут возникать у до 1 из 10 000 человек): панкреатит, реакции с образованием пузырей, очень тяжелые, включая синдром Стивенса-Джонсона (эрозии, распространяющиеся на кожу и две или более слизистые оболочки, и повреждения цвета пурпуры, предпочтительно на туловище) и токсическую эпидермальную некролиз (эрозии на слизистых оболочках и повреждения с некрозом и отслоением эпидермиса), эритема многоформная (повреждение кожи). В случае тяжелой реакции гиперчувствительности общие признаки могут быть отеком лица, языка и гортани, одышкой, тахикардией, гипотензией (анafilaxia, ангioneurotic отек или тяжелый шок), асептический менингит (воспаление оболочек, защищающих мозг и спинной мозг, не вызванное бактериями). В большинстве случаев, когда был зарегистрирован асептический менингит с ибупрофеном, пациент страдал какой-либо формой аутоиммунного заболевания (такого как системный красный волчанка и другие заболевания соединительной ткани), что представляло собой фактор риска. Симптомы асептического менингита, наблюдаемые при этом, были жесткой шеей, головной болью, тошнотой, рвотой, температурой или дезориентацией. Другие очень редкие побочные эффекты включают снижение количества тромбоцитов, снижение количества лейкоцитов (может проявляться как частые инфекции с температурой, ознобом или болью в горле), снижение количества эритроцитов (может проявляться как затруднение дыхания и бледность кожи), нейтропению (снижение нейтрофилов) и агранулоцитоз (очень сильное снижение нейтрофилов), апластическую анемию (недостаточность костного мозга для производства различных типов клеток), гемолитическую анемию (преждевременное разрушение эритроцитов). Первые симптомы: температура, боль в горле, поверхностные язвы во рту, псевдогриппозные симптомы, крайняя усталость, кровотечение и синяки неизвестной причины. Печеночная недостаточность (тяжелое повреждение печени), сердечная недостаточность, инфаркт миокарда, гипертония. Тубулоинтерстициальный нефрит (нарушение функции почек), нефротический синдром (нарушение, характеризующееся белком в моче и отеком тела) и почечная недостаточность (внезапная потеря способности функционировать почек), острый почечный недостаток и некроз сосков (особенно при длительном применении), связанный с увеличением мочевины.
Была зарегистрирована усиление воспалений, связанных с инфекциями, совпадающее с применением НПВС. Если есть признаки инфекции или они ухудшаются во время применения ибупрофена, рекомендуется обратиться к врачу как можно скорее.
- Частота не известна(не может быть оценена на основе доступных данных):
Колит и болезнь Крона (хроническое заболевание, при котором иммунная система атакует кишечник, вызывая воспаление, которое обычно приводит к диарее с кровью).
Общая красная чешуйчатая эрупция, с бугорками под кожей и пузырями, сопровождающаяся температурой. Если вы испытываете эти симптомы, прекратите принимать Ибупрофен Стадафарм и немедленно обратитесь за медицинской помощью. См. также раздел 2.
Кожа становится чувствительной к свету.
Грудная боль, которая может быть признаком потенциально тяжелой аллергической реакции, называемой синдромом Куниса.
Может возникнуть тяжелая кожная реакция, известная как синдром DRESS. Симптомы синдрома DRESS включают: кожную эрупцию, воспаление лимфатических узлов и повышенное количество эозинофилов (тип белых кровяных клеток).
Если出现 любой из следующих побочных эффектов, прекратите лечение и немедленно обратитесь к врачу:
- Аллергические реакции, такие как кожные эрупции, отек лица, шум в груди или затруднение дыхания.
- Рвота с кровью или с видом, подобным кофейной гуще.
- Кровь в кале или диарея с кровью.
- Сильная боль в животе.
- Пузыри или сильная шелушение кожи.
- Сильная головная боль или постоянная.
- Желтый цвет кожи (желтуха).
- Признаки тяжелой гиперчувствительности (аллергии) (см. выше в этом разделе).
- Отек конечностей или накопление жидкости в руках или ногах.
- Красные пятна, не выступающие, в форме мишени или круглые на туловище, часто с пузырями в центре, шелушение кожи, язвы во рту, горле, носу, гениталиях и глазах. Эти тяжелые кожные эрупции могут предшествовать температуре и симптомам, подобным гриппу [эксфолиативный дерматит, эритема полиморфная, синдром Стивенса-Джонсона, токсическая эпидермальная некролиз].
- Общая кожная эрупция, повышенная температура тела и гипертрофия лимфатических узлов (синдром DRESS).
- Общая красная чешуйчатая эрупция, с бугорками под кожей и пузырями, сопровождающаяся температурой. Симптомы обычно появляются в начале лечения (острая генерализованная пустулезная эрупция).
Сообщение о побочных эффектах
Если вы испытываете любой побочный эффект, обратитесь к врачу или фармацевту, даже если это возможные побочные эффекты, не указанные в этом листке-вкладыше. Вы также можете сообщить об этом напрямую через Испанскую систему фармаковигиланса лекарственных средств для человека, www.notificaRAM.es.
5. Хранение Ибупрофена Стадафарма
Храните этот препарат в недоступном для детей месте.
Храните при температуре ниже 30°C.
Не используйте этот препарат после даты истечения срока годности, указанной на упаковке после CAD. Дата истечения срока годности - последний день месяца, указанного.
Лекарства не должны выбрасываться в канализацию или мусор. Поместите упаковку и лекарства, которые вам больше не нужны, в пункт SIGRE аптеки. Если у вас есть сомнения, обратитесь к фармацевту, как избавиться от упаковки и лекарств, которые вам больше не нужны. Таким образом, вы поможете защитить окружающую среду.
6. Содержание упаковки и дополнительная информация
Состав Ибупрофена Стадафарма
- Активное вещество - ибупрофен. Каждый пакет с 10 мл суспензии содержит 400 мг ибупрофена.
- Другие компоненты (вспомогательные вещества) - бензоат натрия (Е-211), глицерин 99,5% (Е-422), жидкий мальтитол (Е-965), камедь ксантановая, сукралоза натрия (Е-954), лимонная кислота безводная, цитрат натрия (Е-331), хлорид натрия, гипромеллоза 15 цПС, клубничный аромат и очищенная вода.
Внешний вид продукта и содержание упаковки
Ибупрофен Стадафарм - это пероральная суспензия белого цвета с клубничным вкусом, содержащаяся в монодозовых пакетах по 10 мл, состоящих из сложного полиэстера, алюминия, полиэстера и полиэтилена.
Препарат выпускается в упаковках по 12 или 20 единиц.
Возможно, не все размеры упаковок будут продаваться.
Владелец разрешения на продажу и ответственный за производство
Владелец разрешения на продажу
Лаборатория STADA, С.Л.
Фредерик Момпу, 5
08960 Сант Хуст Десверн (Барселона)
Испания
Ответственный за производство
АЛКАЛА ФАРМА, С.Л.
Авенида де Мадрид, 82
28802 Алькала де Энарес (Мадрид)
“или”
ЗИНЕРЕО ФАРМА, С.Л.У.
А Рельва, с/н, О Порриньо,
36410 Понтеведра
“или”
ФАРМАЛИДЕР, С.А.
К/Арагонесес, 2
28108 Алькобендас (Мадрид)
“или”
ЕДЕФАРМ С.Л.
Полигоно Индустриаль Энчилагар дель Рулло, 117
46191 Вильямарчанте (Валенсия)
Дата последнего пересмотра этого листка-вкладыша:октябрь 2024
“Детальная и актуальная информация о этом препарате доступна на сайте Агентства по лекарственным средствам и медицинским изделиям Испании (AEMPS) http://www.aemps.gob.es/”
- Страна регистрации
- Активное вещество
- Требуется рецептНет
- Производитель
- Информация носит справочный характер и не является медицинской рекомендацией. Перед приемом любых препаратов проконсультируйтесь с врачом. Oladoctor не несет ответственности за медицинские решения, принятые на основе этого контента.
- Аналоги ИБУПРОФЕН СТАДАФАРМ 400 МГ ОРАЛЬНАЯ СУСПЕНЗИЯФорма выпуска: ОРАЛЬНЫЙ РАСТВОР/СУСПЕНЗИЯ, 600 мг ибупрофенАктивное вещество: ИбупрофенПроизводитель: Laboratorio De Aplicaciones Farmacodinamicas S.A.Требуется рецептФорма выпуска: ОРАЛЬНЫЙ РАСТВОР/СУСПЕНЗИЯ, 200 мгАктивное вещество: ИбупрофенПроизводитель: Laboratorio De Aplicaciones Farmacodinamicas S.A.Требуется рецептФорма выпуска: ОРАЛЬНЫЙ РАСТВОР/СУСПЕНЗИЯ, 20 мг/млАктивное вещество: ИбупрофенПроизводитель: Laboratorio De Aplicaciones Farmacodinamicas S.A.Требуется рецепт
Аналоги ИБУПРОФЕН СТАДАФАРМ 400 МГ ОРАЛЬНАЯ СУСПЕНЗИЯ в других странах
Лучшие аналоги с тем же действующим веществом и терапевтическим эффектом.
Аналог ИБУПРОФЕН СТАДАФАРМ 400 МГ ОРАЛЬНАЯ СУСПЕНЗИЯ в Польша
Аналог ИБУПРОФЕН СТАДАФАРМ 400 МГ ОРАЛЬНАЯ СУСПЕНЗИЯ в Украина
Врачи онлайн по ИБУПРОФЕН СТАДАФАРМ 400 МГ ОРАЛЬНАЯ СУСПЕНЗИЯ
Консультация по дозировке, побочным эффектам, взаимодействиям, противопоказаниям и продлению рецепта на ИБУПРОФЕН СТАДАФАРМ 400 МГ ОРАЛЬНАЯ СУСПЕНЗИЯ – по решению врача и с учетом местных правил.















