
HIBERIX POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE

Cómo usar HIBERIX POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Hiberix polvo y disolvente para solución inyectable
Vacuna conjugada de Haemophilus influenzae tipo b
Lea todo el prospecto detenidamente antes de que su hijo reciba esta vacuna, porque contiene información importante para su hijo.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Esta vacuna se le ha recetado solamente a su hijo y no debe dársela a otras personas.
- Si su hijo experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Hiberix y para qué se utiliza
- Qué necesita saber antes de que su hijo reciba Hiberix
- Cómo se administra Hiberix
- Posibles efectos adversos
- Conservación de Hiberix
- Contenido del envase e información adicional
1. Qué es Hiberix y para qué se utiliza
Hiberix es una vacuna que se utiliza para proteger a su hijo frente a la enfermedad causada por Haemophilus influenzaetipo b.
Haemophilus influenzaetipo b (Hib) puede producir inflamación cerebral. Esto puede dar lugar a problemas graves como: lentitud mental (retardo mental), parálisis cerebral, sordera, epilepsia o ceguera parcial. También puede causar inflamación de la garganta que puede provocar muerte por sofocación. Con menor frecuencia, la bacteria además puede infectar la sangre, el corazón, los pulmones, los huesos, las articulaciones y los tejidos de los ojos y la boca.
Hiberix está indicada en la vacunación primaria de niños a partir de 2 meses de edad incluidos en algunos de los siguientes grupos:
- Niños con asplenia, anemia falciforme o inmunodeficientes.
- Niños infectados con VIH asintomáticos o sintomáticos.
- En aquellas situaciones que determinen las Autoridades Sanitarias correspondientes.
Hiberix ayuda al organismo de su hijo a crear su propia protección (anticuerpos). Esto le protegerá frente a la enfermedad.
Como con todas las vacunas, Hiberix puede no proteger completamente a todos los niños vacunados.
Hiberix sólo protege frente a infecciones causadas por Haemophilus influenzaetipo b, para el que se desarrolló la vacuna.
Puede que los niños que tengan un sistema inmunitario debilitado (debido a una infección por VIH, por ejemplo) no queden completamente protegidos con Hiberix.
La vacuna no puede provocar la enfermedad de la que protege a su hijo.
2. Qué necesita saber antes de que su hijo reciba Hiberix
Hiberix no se debe administrar
- Si su hijo es alérgico (hipersensible) a los principios activos o a cualquiera de los demás componentes de la vacuna (incluidos en la sección 6). Al final del prospecto hay un listado con los principios activos y los otros componentes de Hiberix. Los signos de una reacción alérgica pueden incluir erupción cutánea con picor, dificultad al respirar e inflamación de la cara o la lengua.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de que su hijo reciba Hiberix si:
- su hijo tiene una infección grave con fiebre elevada. En estos casos, la vacunación se pospondrá hasta que se recupere. Una infección menor, como un catarro, no debería constituir un problema, pero consulte primero a su médico
- su hijo tiene dificultad para respirar, informe a su médico. Esto puede ser más frecuente durante los tres primeros días tras la vacunación, si su hijo es prematuro (si ha nacido a las 28 semanas de embarazo o antes).
Antes o después de cualquier inyección podría producirse un desmayo, por lo que debe informar a su médico o enfermera si su hijo se ha desmayado en anteriores ocasiones tras la administración de una inyección.
Uso de Hiberix con otros medicamentos
- Comunique a su médico o farmacéutico que su hijo está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento, incluso los adquiridos sin receta o si le han administrado recientemente alguna otra vacuna.
- Informe a su médico o farmacéutico especialmente si su hijo está utilizando cualquier medicamento o tiene una infección que afecte al sistema inmunitario (el sistema de defensa natural del cuerpo), ya que su hijo puede no quedar completamente protegido con Hiberix.
- Hiberix puede administrarse al mismo tiempo que otras vacunas infantiles. Se utilizarán diferentes lugares de inyección para cada vacuna.
Hiberix no debe mezclarse en la misma jeringa con otras vacunas excepto con Tritanrix HepB.
Hiberix contiene sodio
Este medicamento contiene menos de 23 mg (1mmol) de sodio por 0,5 ml; esto es, esencialmente “exento de sodio”.
3. Cómo se administra Hiberix
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
- Su médico o enfermero administrará la dosis recomendada para su hijo. Ésta dependerá de las recomendaciones oficiales.
- Normalmente, la pauta de vacunación primaria consiste en administrar 3 dosis de Hiberix separadas por un intervalo de 1-2 meses en los 6-7 primeros meses de vida, y puede comenzar a partir de los 2 meses de edad.
- Puede ser necesaria la administración de una dosis adicional (de recuerdo) en el segundo año de vida, como por ejemplo en aquellos niños que no hayan completado la vacunación primaria. Si es necesaria, su médico o enfermera se lo comunicarán.
- Hiberix se administra en el músculo.
- La vacuna nunca debe administrarse en una vena.
- Se le informará de cuándo se le debe administrar a su hijo la siguiente dosis.
Si se administra más Hiberix de la que se debiera
No se ha comunicado ningún caso de sobredosis. Dado que el envase contiene sólo una dosis, es poco probable que se produzca una sobredosis.
Si su hijo no recibe una dosis de Hiberix
- Si su hijo no recibe una dosis prevista, es importante que pida otra cita. En caso de que la administración de una dosis no se realizase de acuerdo con el calendario previsto, se puede retrasar la administración de ésta, siempre que las 3 dosis se administren durante el primer año de vida manteniendo un intervalo de 1-2 meses entre las dosis.
- Si no termina el ciclo completo de vacunación de las tres inyecciones, puede que su hijo no obtenga la mejor respuesta inmunológica ni protección frente a la enfermedad.
4. Posibles efectos adversos
Al igual que todos los medicamentos, Hiberix puede producir efectos adversos, aunque no todas las personas los sufran.
Los efectos adversos que tuvieron lugar durante los ensayos clínicos fueron los siguientes:
Reacciones alérgicas
Como con todas las vacunas inyectables, su hijo puede experimentar una reacción alérgica, aunque éstas son muy raras (menos de 1 por cada 10.000 dosis de vacuna).
Los signos de una reacción alérgica pueden ser:
- erupciones cutáneas que pueden producir picor o vesículas
- hinchazón de los ojos y la cara
- dificultad para respirar o tragar
- repentina bajada de la presión sanguínea
- pérdida de consciencia
Normalmente estos síntomas aparecen inmediatamente tras la inyección. Lleve inmediatamente a su hijo al médico si estos comienzan al salir de la clínica.
Acuda al médico enseguida si su hijo tiene cualquiera de los siguientes efectos adversos graves
Muy frecuentes(pueden ocurrir en más de 1 de cada 10 dosis de la vacuna):
- irritabilidad
- somnolencia
- fiebre
- hinchazón, dolor y enrojecimiento en el lugar de la inyección
- pérdida de apetito
- llanto
- inquietud
- diarrea
Frecuentes(pueden ocurrir hasta en 1 de cada 10 dosis de la vacuna):
- vómitos
Raras(pueden ocurrir hasta en 1 de cada 1.000 dosis de la vacuna):
- ataques (incluyendo ataques debido a la fiebre)
Adicionalmente, otros efectos no observados durante los ensayos clínicos, pero notificados tras la comercialización de Hiberix, son:
Muy raros(pueden ocurrir en menos de 1 por cada 10.000 dosis de vacuna):
- desmayo debido a la inyección
- colapso (pérdida repentina del tono muscular), períodos de inconsciencia o pérdida del conocimiento y palidez o coloración azulada de la piel
- interrupción temporal de la respiración
- urticaria, erupción cutánea localizada en una o varias zonas, o en todo el cuerpo
- hinchazón de la extremidad donde se ha inyectado la vacuna
- bulto duro en el lugar de inyección
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano:
www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Hiberix
- Mantener este medicamento fuera de la vista y del alcance de los niños.
- Conservar en nevera (entre 2ºC y 8ºC).
- No congelar.
- Conservar en el embalaje original para protegerlo de la luz.
- No utilice Hiberix después de la fecha de caducidad que aparece en el envase. La fecha de caducidad es el último día del mes que se indica.
- Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e informacion adicional
Composición de Hiberix
- Los principios activos son:
Polisacárido de Haemophilus influenzaetipo b 10 microgramos
conjugado con toxoide tetánico como proteína transportadora aprox. 25 microgramos
- Los demás componentes son:
Polvo: lactosa
Disolvente: cloruro de sodio y agua para preparaciones inyectables
Aspecto del producto y contenido del envase
Hiberix se presenta como un polvo en un vial de vidrio y un disolvente en una jeringa precargada.
El polvo es blanco y el disolvente es transparente e incoloro.
Titular de la autorización de comercialización
GlaxoSmithKline, S.A.
PTM - C/ Severo Ochoa, 2
28760 Tres Cantos
Madrid
Teléfono: 900 202 700
e-mail: [email protected]
Responsable de la fabricación
GlaxoSmithKline Biologicals S.A.
Rue de L’Institut 89; 1330 Rixensart
Bélgica
ó
SMITHKLINE BEECHAM, S.A.
Ctra. de Ajalvir Km. 2,5. (Alcalá de Henares (Madrid)) – 28806
España
Fecha de la última revisión de este prospecto:12/2019
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/.
---------------------------------------------------------------------------------------------------------------
Esta información está destinada únicamente a profesionales del sector sanitario:
Antes de la reconstitución o administración, se deben inspeccionar visualmente el disolvente y la vacuna reconstituida para detectar si existe cualquier partícula extraña y/o variación del aspecto físico. Si se observa cualquiera de ellas, no usar el disolvente o la vacuna reconstituida.
Instrucciones para la reconstitución de la vacuna con el disolvente en jeringa precargada
Hiberix se debe reconstituir añadiendo todo el contenido de la jeringa precargada al vial que contiene el polvo.
Para saber cómo insertar la aguja en la jeringa, léanse detenidamente las instrucciones proporcionadas con las imágenes 1 y 2. No obstante, la jeringa facilitada con Hiberix puede ser ligeramente diferente (sin rosca de tornillo) a la jeringa de la imagen. En tal caso, la aguja deberá insertarse sin enroscar.
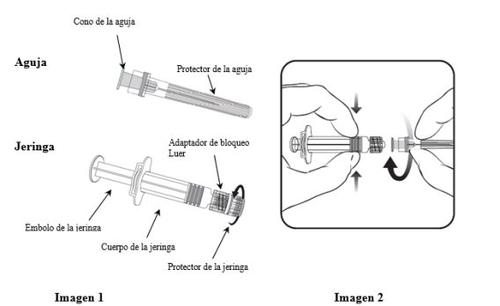
Sujetar siempre la jeringa por el cuerpo, no por el émbolo ni por el adaptador de bloqueo Luer (ABL), y mantener la aguja en el eje de la jeringa (como se muestra en la imagen 2). De lo contrario, el ABL podría deformarse y causar fugas.
Si durante el ensamblaje de la jeringa se desprende el ABL, usar una nueva dosis de la vacuna (nueva jeringa y vial).
- Desenroscar el protector de la jeringa girándolo en sentido contrario a las agujas del reloj (como se muestra en la imagen 1).
Tanto si el ABL gira como si no, por favor, siga los siguientes pasos:
- Insertar la aguja en la jeringa encajando con delicadeza el cono de la aguja en el ABL y girar un cuarto de vuelta en el sentido de las agujas del reloj hasta sentir que se bloquea (como se muestra en la imagen 2).
- Retirar el protector de la aguja (puede resultar difícil).
- Añadir el disolvente al polvo. Se debe agitar bien la mezcla hasta que el polvo esté completamente disuelto.
La vacuna reconstituida es una solución transparente a opalescente e incolora.
Tras la reconstitución, la vacuna debe administrarse rápidamente. Si no se utiliza a las 8 horas tras la reconstitución, deberá desecharse.
- Se debe utilizar una aguja nueva para administrar la vacuna. Desenroscar la aguja de la jeringa e insertar la aguja para la inyección repitiendo el paso 2.
Hiberix puede mezclarse en la misma jeringa con la vacuna monodosis Tritanrix HepB. Se debe comprobar que la vacuna que se va a mezclar con Hiberix se presente en envase monodosis. Del embalaje de Hiberix, descarte el recipiente que contiene el disolvente. En este caso, el disolvente incluido en el embalaje de Hiberix se sustituirá por la vacuna líquida de Tritanrix HepB. La vacuna combinada debe reconstituirse añadiendo el contenido completo del recipiente de Tritanrix HepB al vial que contiene el polvo blanco de Hib. Esta vacuna combinada extemporáneamente debería manejarse del mismo modo que la vacuna monocomponente reconstituida Hiberix.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a HIBERIX POLVO Y DISOLVENTE PARA SOLUCION INYECTABLEForma farmacéutica: INYECTABLE, 0,5 ml dosis únicaPrincipio activo: meningococcus B, multicomponent vaccineFabricante: Glaxosmithkline Vaccines S.R.L.Requiere recetaForma farmacéutica: INYECTABLE, 0,5 ml dosis únicaPrincipio activo: meningococcus B, multicomponent vaccineFabricante: Glaxosmithkline Vaccines S.R.L.Requiere recetaForma farmacéutica: INYECTABLE, -Principio activo: pertussis, purified antigen, combinations with toxoidsFabricante: Glaxosmithkline S.A.Requiere receta
Médicos online para HIBERIX POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de HIBERIX POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















