GONAL-F 150 UI/0,24 ML SOLUCION INYECTABLE EN PLUMA PRECARGADA

Cómo usar GONAL-F 150 UI/0,24 ML SOLUCION INYECTABLE EN PLUMA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
GONAL-f 150 UI/0,24ml solución inyectable en pluma precargada
folitropina alfa
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es GONAL-f y para qué se utiliza
- Qué necesita saber antes de empezar a usar GONAL-f
- Cómo usar GONAL-f
- Posibles efectos adversos
- Conservación de GONAL-f
- Contenido del envase e información adicional
Instrucciones de uso
1. Qué es GONAL-f y para qué se utiliza
Qué es GONAL-f
GONAL-f contiene una sustancia llamada “folitropina alfa”. La folitropina alfa es un tipo de ‘hormona foliculoestimulante’ (FSH), que pertenece a la familia de hormonas llamadas ‘gonadotropinas’. Las gonadotropinas intervienen en la reproducción y la fertilidad.
Para qué se utiliza GONAL-f
En mujeres adultas,GONAL-f se utiliza:
- para ayudar a liberar un óvulo del ovario (ovulación) en mujeres que no pueden ovular y que no han respondido al tratamiento con una sustancia llamada ‘citrato de clomifeno’.
- junto con otra sustancia llamada ‘lutropina alfa’ (‘hormona luteinizante’ o LH), para ayudar a liberar un óvulo del ovario (ovulación) en mujeres cuyo organismo produce cantidades muy pequeñas de gonadotropinas (FSH y LH).
- para ayudar a desarrollar varios folículos (en que cada uno contiene un óvulo) en mujeres que se someten a técnicas de reproducción asistida (técnicas que pueden ayudarla a quedarse embarazada), como la ‘fertilización in vitro’, la ‘transferencia intratubárica de gametos’ o la ‘transferencia intratubárica de cigotos’.
En varones adultos,GONAL-f se utiliza:
- junto con otra sustancia llamada ‘gonadotropina coriónica humana’ (hCG), para ayudar a producir esperma en varones que son infértiles debido a una concentración baja de ciertas hormonas.
2. Qué necesita saber antes de empezar a usar GONAL-f
Antes de iniciar el tratamiento debe valorarse su fertilidad y la de su pareja por parte de un médico experimentado en el tratamiento de los trastornos de la fertilidad.
No use GONAL-f
- si es alérgico a la hormona foliculoestimulante o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- si tiene un tumor en el hipotálamo o en la hipófisis (ambos son partes del cerebro).
- si usted es una mujer:
- con ovarios grandes o bolsas de líquido en el interior de los ovarios (quistes ováricos) de origen desconocido.
- con hemorragia vaginal inexplicada.
- con cáncer de ovario, de útero o de mama.
- si tiene una afección que normalmente hace que el embarazo sea imposible, como la insuficiencia ovárica (menopausia precoz) o una malformación de los órganos reproductivos.
- si usted es un varón:
- con testículos dañados que no pueden curarse.
No utilice GONAL-f si alguna de las condiciones anteriores le aplica a usted. Si no está seguro, consulte a su médico antes de empezar a usar este medicamento.
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de empezar a usar GONAL-f.
Porfiria
Informe a su médico antes de iniciar el tratamiento, si usted o algún miembro de su familia padece porfiria (una incapacidad para degradar las porfirinas que puede transmitirse de padres a hijos).
Informe inmediatamente a su médico si:
- su piel se vuelve frágil y le salen ampollas con facilidad, especialmente en las zonas expuestas al sol con frecuencia, y/o
- si tiene dolor de estómago, de brazos o piernas.
En estos casos, su médico puede recomendarle que interrumpa el tratamiento.
Síndrome de hiperestimulación ovárica (SHO)
Si es usted una mujer, este medicamento aumenta el riesgo de que presente un SHO. Esto ocurre cuando sus folículos se desarrollan demasiado y se convierten en quistes de gran tamaño. Si tiene dolor en la región pélvica, aumenta de peso rápidamente, tiene náuseas o vómitos o dificultad para respirar, consulte inmediatamente con su médico, quien puede interrumpir el tratamiento (ver la sección 4).
En caso de que no ovule y se respeten la dosis y el esquema posológico recomendados, ese síndrome es menos probable que ocurra. El tratamiento con GONAL-f rara vez causa un síndrome de hiperestimulación ovárica grave, a menos que se administre el medicamento que se usa para la maduración folicular final (que contiene gonadotropina coriónica humana, hCG). En caso de desarrollar SHO, su médico puede no recetarle hCG en este ciclo de tratamiento y aconsejarle que se abstenga de realizar el coito o que utilice métodos anticonceptivos de barrera durante al menos 4 días.
Embarazo múltiple
Si usa GONAL-f, tiene un riesgo más alto de quedarse embarazada de más de un niño a la vez (‘embarazo múltiple’, generalmente gemelos), que si se queda embarazada por concepción natural. El embarazo múltiple puede causar complicaciones médicas para usted y sus bebés. Usted puede reducir el riesgo de embarazo múltiple usando la dosis correcta de GONAL-f a las horas correctas. Si se somete a técnicas de reproducción asistida, el riesgo de embarazo múltiple está relacionado con su edad y con la calidad y el número de óvulos fertilizados o embriones que se coloquen en su interior.
Aborto
Si se somete a técnicas de reproducción asistida o a estimulación de sus ovarios para producir óvulos, la probabilidad de tener un aborto es mayor que en el promedio de las mujeres.
Problemas de coagulación de la sangre (episodios tromboembólicos)
Si usted o algún miembro de su familia ha sufrido, en el pasado o recientemente, coágulos de sangre en la pierna o en el pulmón, infarto de miocardio o ictus, usted podría tener un riesgo más alto de presentar estos problemas o de que empeorasen con el tratamiento con GONAL-f.
Varones con niveles altos de FSH en la sangre
Si usted es varón, unos niveles demasiado altos de FSH en la sangre pueden ser un signo de lesión de los testículos. Por lo general, GONAL-f no suele ser eficaz en estos casos.
Si su médico decide intentar el tratamiento con GONAL-f, para controlar el tratamiento, su médico puede pedirle que se haga un análisis de semen, de 4 a 6 meses después de iniciar el tratamiento.
Niños y adolescentes
GONAL-f no debe utilizarse en niños y adolescentes menores de 18 años de edad.
Otros medicamentos y GONAL-f
Informe a su médico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento.
- Si usa GONAL-f con otros medicamentos que ayudan a la ovulación (por ejemplo, hCG o citrato de clomifeno), la respuesta de sus folículos puede verse aumentada.
- Si usa GONAL-f al mismo tiempo que un agonista o un antagonista de la ‘hormona liberadora de gonadotropinas’ (GnRH) (estos medicamentos disminuyen las concentraciones de las hormonas sexuales y detienen la ovulación), puede necesitar una dosis más alta de GONAL-f para producir folículos.
Embarazo y lactancia
No use GONAL-f si está embarazada o en periodo de lactancia.
Conducción y uso de máquinas
No se espera que este medicamento afecte a su capacidad para conducir y utilizar máquinas.
GONAL-f contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por dosis; esto es, esencialmente “exento de sodio”.
3. Cómo usar GONAL-f
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Uso de este medicamento
- GONAL-f está diseñado para que se administre mediante inyección justo debajo de la piel (por vía subcutánea). La pluma precargada puede usarse para varias inyecciones.
- La primera inyección de GONAL-f debe administrarse bajo la supervisión de su médico.
- Su médico o enfermero le enseñarán cómo usar la pluma precargada de GONAL-f para inyectarse el medicamento.
- Si usted se autoadministra GONAL-f, lea y siga atentamente las “Instrucciones de uso”.
Qué cantidad se debe usar
Su médico decidirá qué cantidad de medicamento se administrará y con qué frecuencia. Las dosis que se describen a continuación están expresadas en Unidades Internacionales (UI).
Mujeres
Si no está ovulando y tiene menstruaciones irregulares o no tiene menstruación.
- GONAL-f se administra generalmente todos los días.
- Si usted tiene una menstruación irregular, empiece usando GONAL-f en los 7 primeros días del ciclo menstrual. Si no tiene menstruación, puede empezar a usar el medicamento cualquier día que le resulte cómodo.
- La dosis inicial de GONAL-f suele individualizarse y puede ajustarse de forma escalonada.
- La dosis diaria de GONAL-f no debe ser superior a 225 UI.
- Cuando se obtenga la respuesta deseada, se le administrará una inyección única de 250 microgramos de ‘hCG recombinante’ (r-hCG, una hCG fabricada en un laboratorio mediante una técnica especial de ADN), o de 5 000 a 10 000 UI de hCG, 24 a 48 horas después de la última inyección de GONAL-f. El mejor momento para mantener relaciones sexuales es el mismo día de la inyección de hCG y al día siguiente.
Si su médico no observa la respuesta deseada después de 4 semanas de tratamiento, ese ciclo de tratamiento con GONAL-f debe interrumpirse. Para el siguiente ciclo, su médico le administrará una dosis inicial más alta de GONAL-f que la anterior.
Si se obtiene una respuesta excesiva, se interrumpirá su tratamiento y no le administrará hCG (ver la sección 2, Síndrome de hiperestimulación ovárica (SHO)”). Para el siguiente ciclo, su médico le administrará una dosis de GONAL-f más baja que la del ciclo previo.
Si se le han diagnosticado niveles muy bajos de hormonas FSH y LH
- La dosis inicial habitual de GONAL-f es de 75 a 150 UI, junto con 75 UI de lutropina alfa.
- Usará estos dos medicamentos cada día, hasta un período de cinco semanas.
- La dosis de GONAL-f puede aumentarse cada 7 o 14 días, en 37,5 a 75 UI, hasta que se obtenga la respuesta deseada.
- Cuando se obtenga la respuesta deseada, se le administrará una inyección única de 250 microgramos de ‘hCG recombinante’ (r-hCG, una hCG fabricada en un laboratorio mediante una técnica especial de ADN), o de 5 000 a 10 000 UI de hCG, 24 a 48 horas después de la última inyección de GONAL-f y lutropina alfa. El mejor momento para mantener relaciones sexuales es el mismo día de la inyección de hCG y al día siguiente. Como alternativa, también, puede realizarse una inseminación intrauterina u otro procedimiento de reproducción médicamente asistida, a criterio de su médico.
Si su médico no observa la respuesta deseada después de cinco semanas, ese ciclo de tratamiento con GONAL-f debe interrumpirse. Para el siguiente ciclo, su médico le administrará una dosis inicial más alta de GONAL-f que la del ciclo cancelado.
Si se obtiene una respuesta excesiva, se interrumpirá su tratamiento y no le administrarán hCG (ver la sección 2, “Síndrome de hiperestimulación ovárica (SHO)”). Para el siguiente ciclo, su médico le administrará una dosis de GONAL-f más baja que la del ciclo previo.
Si tiene que desarrollar varios óvulos para su extracción previamente a cualquier técnica de reproducción asistida
- La dosis inicial de GONAL-f suele individualizarse y puede ajustarse de forma escalonada has un máximo de 450 UI al día.
- El tratamiento continúa hasta que los óvulos se hayan desarrollado hasta el punto deseado. Su médico lo comprobará mediante análisis de sangre y/o ecografías.
- Cuando los óvulos estén listos, se le administrará una inyección única de 250 microgramos de ‘hCG recombinante’ (r-hCG, una hCG fabricada en un laboratorio mediante una técnica especial de ADN recombinante), o de 5 000 UI a 10 000 UI de hCG, 24 a 48 horas después de la última inyección de GONAL-f. Esto hace que sus óvulos estén listos para su extracción.
Varones
- La dosis habitual de GONAL-f es de 150 UI junto con hCG.
- Usted usará estos dos medicamentos tres veces por semana, durante al menos 4 meses.
- Si no ha respondido al tratamiento después de 4 meses, su médico puede sugerirle que siga usando estos dos medicamentos al menos durante 18 meses.
Si usa más GONAL-f del que debe
Se desconocen los efectos de usar una cantidad excesiva de GONAL-f. Sin embargo, puede esperarse que se produzca un síndrome de hiperestimulación ovárica (SHO), que se describe en la sección 4. Sin embargo, este síndrome sólo ocurrirá si se administra también hCG (ver la sección 2, “Síndrome de hiperestimulación ovárica (SHO)”).
Si olvidó usar GONAL-f
Si olvidó usar GONAL-f, no tome una dosis doble para compensar las dosis olvidadas. Consulte a su médico tan pronto como se dé cuenta de que se ha olvidado de tomar una dosis.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Efectos adversos graves en mujeres
- El dolor pélvico, acompañado de náuseas o vómitos, pueden ser síntomas del síndrome de hiperestimulación ovárica (SHO). Esto puede indicar que los ovarios han reaccionado de forma excesiva al tratamiento y se han desarrollado quistes ováricos de gran tamaño (ver sección 2, en “Síndrome de hiperestimulación ovárica (SHO)”). Este efecto adverso es frecuente (puede afectar hasta 1 de cada 10 personas).
- El síndrome de hiperestimulación ovárica puede agravarse con ovarios claramente aumentados de tamaño, disminución de la producción de orina, aumento de peso, dificultad para respirar y/o posible acumulación de líquido en el abdomen o en el pecho. Este efecto adverso es poco frecuente (puede afectar hasta 1 de cada 100 personas).
- En casos raros, también pueden producirse complicaciones del síndrome de hiperestimulación ovárica como torsión ovárica o coagulación de la sangre (puede afectar hasta 1 de cada 1 000 personas).
- En casos muy raros (pueden afectar hasta 1 de cada 10 000 personas) pueden producirse complicaciones graves de la coagulación de la sangre (episodios tromboembólicos), a veces independientes del síndrome de hiperestimulación ovárica. Esto podría causar dolor en el pecho, sensación de falta de aire, ictus o infarto de miocardio (ver también sección 2, en “Problemas de coagulación de la sangre”).
Efectos adversos graves en varones y en mujeres
- Las reacciones alérgicas, como erupción cutánea, enrojecimiento de la piel, ampollas, hinchazón de la cara con dificultad para respirar, a veces pueden ser graves. Este efecto adverso es muy raro (puede afectar hasta 1 de cada 10 000 personas).
Si observa alguno de los efectos adversos antes mencionados, debe consultar inmediatamente a su médico, quien podría pedirle que interrumpa el tratamiento con GONAL-f.
Otros efectos adversos en mujeres
Muy frecuentes (pueden afectar a más de 1 de cada 10 personas):
- Bolsas de líquido en el interior de los ovarios (quistes ováricos).
- Dolor de cabeza.
- Reacciones locales en el lugar de la inyección, como dolor, enrojecimiento, hematoma, hinchazón y/o irritación.
Frecuentes (pueden afectar hasta 1 de cada 10 personas):
- Dolor abdominal.
- Náuseas, vómitos, diarrea, retortijones y flatulencias.
Muy raros (pueden afectar hasta 1 de cada 10 000 personas):
- Pueden producirse reacciones alérgicas, como erupción cutánea, enrojecimiento de la piel, ampollas, hinchazón de la cara con dificultad para respirar. En ocasiones, estas reacciones pueden ser graves.
- El asma puede empeorar.
Otros efectos adversos en varones
Muy frecuentes (pueden afectar a más de 1 de cada 10 personas):
- Reacciones locales en el lugar de la inyección, como dolor, enrojecimiento, hematoma, hinchazón y/o irritación.
Frecuentes (pueden afectar hasta 1 de cada 10 personas):
- Hinchazón de las venas por encima y por detrás de los testículos (varicocele).
- Desarrollo de mamas, acné o aumento de peso.
Muy raros (pueden afectar hasta 1 de cada 10 000 personas):
- Pueden producirse reacciones alérgicas, como erupción cutánea, enrojecimiento de la piel, ampollas, hinchazón de la cara con dificultad para respirar. En ocasiones, estas reacciones pueden ser graves.
- El asma puede empeorar.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunircarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de GONAL-f
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta del cartucho o en la caja después de CAD. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2ºC y 8ºC). No congelar.
Antes de la apertura y dentro de su periodo de validez, el producto puede conservarse fuera de la nevera a temperaturas de hasta un máximo de 25ºC durante un periodo único máximo de 3 meses y debe desecharse si no se ha utilizado en estos 3 meses.
Conservar el capuchón colocado en la pluma para protegerla de la luz.
No utilice GONAL-f si observa cualquier indicio visible de deterioro, si el líquido contiene partículas o si no es transparente.
Una vez abierta, la pluma debe conservarse entre 2ºC y 25ºC durante un máximo de 28 días.
No use el medicamento que permanezca en la pluma precargada más de 28 días.
Al final del tratamiento, la solución no utilizada debe desecharse.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adiciona
Composición de GONAL-f
- El principio activo es folitropina alfa.
- Hay 600 UI (44 microgramos) de folitropina alfa en cada mililitro de líquido. Cada pluma precargada con cartucho multidosis proporciona 150 UI (11 microgramos) en 0,25 ml.
- Los demás componentes son poloxámero 188, sacarosa, metionina, dihidrogenofosfato de sodio monohidrato, hidrogenofosfato de disodio dihidrato y m-cresol, así como ácido fosfórico concentrado e hidróxido de sodio para ajuste de pH y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
- GONAL-f se presenta como un líquido inyectable, transparente e incoloro, en pluma precargada.
- Se suministra en cajas con 1 pluma precargada y 4 agujas desechables.
Titular de la autorización de comercialización
Merck Europe B.V., Gustav Mahlerplein 102, 1082 MA Amsterdam, Países Bajos
Responsable de la fabricación
Merck Serono S.p.A., Via delle Magnolie 15, 70026 Modugno (Bari), Italia
Fecha de la última revisión de este prospecto: {MM/AAAA}.
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu/.
Instrucciones de uso
GONAL-f PLUMA PRECARGADA 150 UI/0,24ml
Solución inyectable en pluma precargada
Folitropina alfa
Información importante sobre la pluma precargada de GONAL-f
- Lea las instrucciones de uso y el prospecto antes de usar la pluma precargada de GONAL-f.
- Siga siempre todas las indicaciones de estas instrucciones de uso y la formación que le ha proporcionado el profesional sanitario, ya que pueden ser distintas de las recibidas con anterioridad. Esta información permitirá evitar errores en el tratamiento o infecciones por pinchazo de aguja o lesiones por rotura del vidrio.
- La pluma precargada de GONAL-f es solo para inyección por vía subcutánea.
- Solo use la pluma precargada de GONAL-f si el profesional sanitario le enseña cómo usarla correctamente.
- El profesional sanitario le dirá cuántas plumas precargadas de GONAL-f necesita para completar su tratamiento.
- Póngase la inyección a la misma hora cada día.
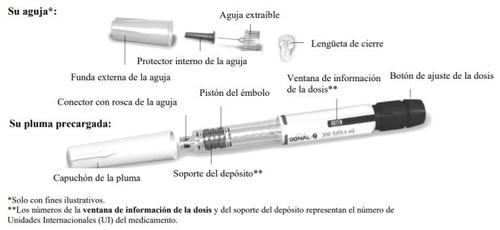
- Los números de la ventana de información de la dosisrepresentan el número de unidades internacionales (UI) y muestran la dosis de folitropina alfa. El profesional sanitario le dirá cuántas UI de folitropina alfa se debe inyectar cada día.
- Los números mostrados en la ventana de información de la dosisle ayudan a:
|
|
|
|
|
|
- Retire la aguja de la pluma inmediatamente después de cada inyección.
Noreutilice las agujas.
Nocomparta la pluma ni las agujas con ninguna otra persona.
Noutilice la pluma precargada de GONAL-f si se ha caído, o si la pluma está agrietada o dañada, ya que esto puede causar lesiones.
Cómo usar el diario de tratamiento de la pluma precargada de GONAL-f
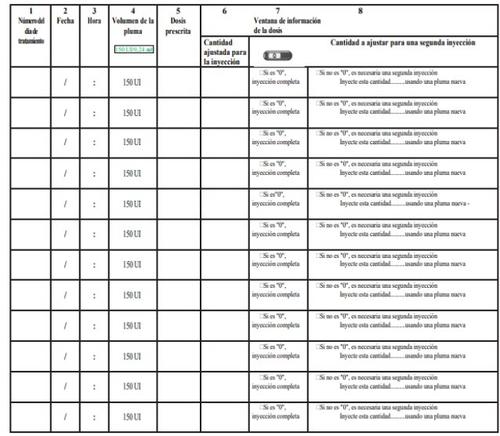
Al final de las instrucciones de uso se incluye un diario de tratamiento. Use el diario de tratamiento para anotar la cantidad inyectada.
Inyectar una cantidad incorrecta de medicamento puede afectar al tratamiento.
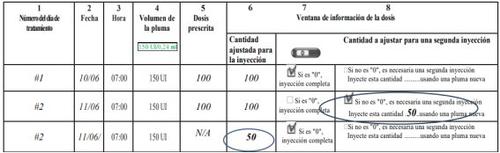
- Anote el número del día de tratamiento (columna 1), la fecha (columna 2), la hora de la inyección (columna 3) y el volumen de la pluma (columna 4).
- Anote la dosis que le han prescrito (columna 5).
- Compruebe que ha seleccionado la dosis correcta antes de efectuar la inyección (columna 6).
- Después de la inyección, lea el número indicado en la ventana de información de la dosis.
- Confirme que ha recibido una inyección completa (columna 7) o anote el número indicado en la ventana de información de la dosis si es distinto de “0” (columna 8).
- Cuando sea necesario, realice otra inyección con una segunda pluma, seleccionando la dosis restante que aparece escrita en la sección “Cantidad a ajustar para una segunda inyección” (columna 8).
- Anote esta dosis restante en la sección “Cantidad ajustada para la inyección”(columna 6) en la siguiente línea.
El uso del diario de tratamiento para anotar la(s) inyección(es) diaria(s) le permite comprobar que ha recibido la dosis prescrita completa cada día.
Un ejemplo de diario de tratamiento:
Familiarícese con la pluma precargada de GONAL-f
Paso 1 Reúna los materiales
1.1 Deje la pluma precargada a temperatura ambiente durante al menos 30 minutos antes de usarla para permitir que el medicamento alcance la temperatura ambiente. Nouse un microondas ni ningún otro elemento calefactor para calentar la pluma. 1.2 Prepare un lugar limpio y una superficie plana, como una mesa o encimera, en una zona bien iluminada. 1.3 También necesitará (no incluidos en el envase):
1.4 Lávese las manos con agua y jabón y séquelas bien a continuación (Figura 5). 1.5 Extraiga con la mano la pluma precargada de GONAL-f del envase. Noutilice ningún utensilio, ya que su uso puede dañar la pluma. 1.6 Compruebe que sobre la pluma precargada ponga GONAL-f. 1.7 Compruebe la fecha de caducidad en la etiqueta de la pluma (Figura 6). Noutilice la pluma precargada de GONAL-f si ya ha pasado la fecha de caducidad o si en la pluma precargada no pone GONAL-f |
|
Paso 2 Prepárese para la inyección
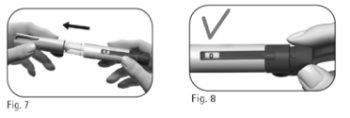
2.1 Retire el capuchón de la pluma (Figura 7). 2.2 Compruebe que el medicamento es transparente e incoloro y no contiene partículas. Noutilice la pluma precargada si el medicamento ha cambiado de color o está turbio, ya que esto puede causar una infección. 2.3 Compruebe que la ventana de información de la dosis está ajustada a “0” (Figura 8). |
|
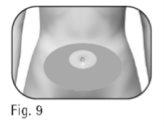
Elija un lugar de inyección: 2.4 El profesional sanitario deberá indicarle los lugares de inyección que debe utilizar alrededor de la zona del estómago (Figura 9). Para reducir al mínimo la irritación cutánea, escoja un lugar de inyección diferente cada día. 2.5 Limpie la piel del lugar de inyección con una toallita empapada en alcohol. Notoque ni cubra la piel que acaba de limpiar. |
|
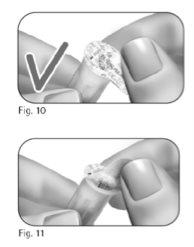
Paso 3 Acople la aguja
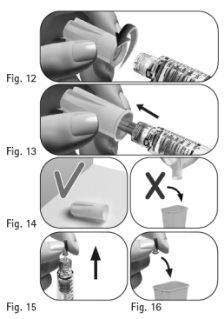
Importante:asegúrese siempre de utilizar una aguja nueva para cada inyección. Reutilizar las agujas puede causar una infección. 3.1 Tome una nueva aguja. Utilice solamente las agujas “de un solo uso” suministradas. 3.2 Compruebe que la funda externa de la aguja no esté dañada. 3.3 Sujete con firmeza la funda externa de la aguja. 3.4 Compruebe que la lengüeta de cierre de la funda externa de la aguja no esté dañada o suelta y que no ha pasado la fecha de caducidad (Figura 10). 3.5 Retire la lengüeta de cierre (Figura 11). Noutilice la aguja si está dañada o caducada o si la funda externa de la aguja o la lengüeta de cierre está dañada o suelta. Utilizar agujas caducadas o agujas con lengüetas de cierre o fundas externas de la aguja dañadas puede causar una infección. Tírela en un contenedor para objetos cortantes y punzantes y tome una aguja nueva. 3.6 Enrosque la funda externa de la aguja en la punta con rosca de la pluma precargada de GONAL-f hasta que note una ligera resistencia (Figura 12). Noapriete demasiado la aguja al acoplarla, ya que podría ser difícil extraerla después de la inyección. 3.7 Retire la funda externa de la aguja tirando de ella suavemente (Figura 13). 3.8 Déjela a un lado para usarla después (Figura 14). |
|
Nodeseche la funda externa de la aguja, ya que esta evitará lesiones por pinchazo con la aguja e infecciones al separar la aguja de la pluma precargada. 3.9 Sostenga la pluma precargada de GONAL-f con la aguja apuntando hacia arriba (Figura 15). 3.10 Retire cuidadosamente y deseche el protector interno de color verde de la aguja (Figura 16). Novuelva a tapar la aguja con el protector interno de color verde de la aguja, ya que esto puede provocar lesiones por pinchazo con la aguja e infecciones. 3.11 Examine minuciosamente la punta de la aguja en busca de una o varias gotitas de líquido. |
| |
Si | Entonces | |
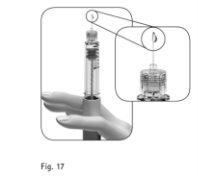
Utiliza una pluma nueva | Compruebe que haya una gotita de líquido en la punta de la aguja(Figura 17).
| |
Reutiliza una pluma | NO es necesario comprobar que haya una gotita de líquido. Procedadirectamente con el Paso 4 Seleccione la dosis. |
Si no observa ninguna gotita de líquido en la punta o sus proximidades la primera vez que utilice una pluma nueva:

- Gire con cuidado el botón de ajuste de la dosis hacia delante hasta que indique “25” en la ventana de información de la dosis(Figura 18).
- Puede girar el botón de ajuste de la dosis hacia atrás si lo ha desplazado más allá de “25”.
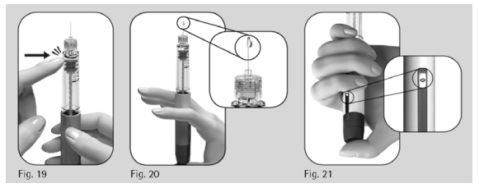
- Sostenga la pluma con la aguja apuntando hacia arriba.
- Golpee suavemente el soporte del depósito (Figura 19).
- Pulse el botón de ajuste de la dosis por completo. Aparecerá una gotita de líquido en la punta de la aguja (Figura 20).
- Compruebe que la ventana de información de la dosisindica “0” (Figura 21).
- Proceda con el Paso 4 Seleccione la dosis.
Si no aparece una gotita de líquido, póngase en contacto con el profesional sanitario.
Paso 4 Seleccione la dosis
Nota:La pluma contiene 150 UI de folitropina alfa. La dosis única máxima que se puede ajustar en una pluma de 150 UI es 150 UI. La dosis única mínima es 12,5 UI y la dosis puede aumentarse en incrementos de 12,5 UI.
4.1.Gire el botón de ajuste de la dosis hasta que la dosis deseada aparezca en la ventana de información de la dosis
- Ejemplo: si la dosis deseada es “150” UI, confirme que la ventana de información de la dosis muestra “150” (Figura 22). Inyectar una cantidad incorrecta de medicamento puede afectar al tratamiento.
|
|
|
|
4.2.Compruebe que la ventana de información de la dosisindica la dosis prescrita completaantes de continuar con el siguiente paso.
Paso 5 Inyecte la dosis
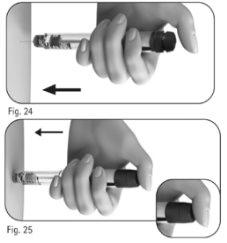
Importante:inyecte la dosis tal como le ha enseñado el profesional sanitario. 5.1 Inserte lentamente la totalidad de la aguja en la piel (Figura 24). 5.2 Coloque el pulgar en el centro del botón de ajuste de la dosis. Apriete el botón de ajuste de la dosis lenta y completamentey manténgalo apretado para administrar íntegramente la inyección (Figura 25). Nota:cuanto mayor sea la dosis, más tiempo llevará inyectarla. |
|
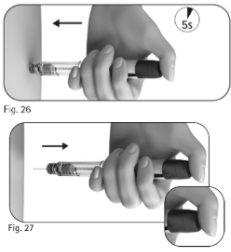
5.3 Mantenga el botón de ajuste de la dosis apretado durante un mínimo de 5 segundos antes de extraer la aguja de la piel (Figura 26).
Nosuelte el botón de ajuste de la dosis hasta haber retirado la aguja de la piel. |
|
Paso 6 Retire la aguja después de cada inyección
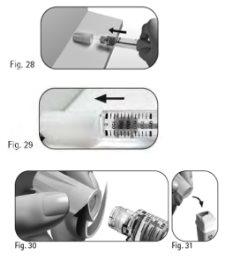
6.1 Coloque la funda externa de la aguja sobre una superficie plana. 6.2 Sostenga la pluma precargada de GONAL-f firmemente con una mano e introduzca la aguja en la funda externa de la aguja (Figura 28). 6.3 Continúe empujando la aguja enfundada contra una superficie firme hasta que oiga un chasquido (“clic”) (Figura 29). 6.4 Sujete la funda externa de la aguja y desenrosque la aguja girándola en la dirección opuesta (Figura 30). 6.5 Deseche la aguja usada de forma seguraen un contenedor para objetos cortantes y punzantes (Figura 31). Manipule la aguja con cuidado para evitar lesionarse con ella. Noreutilice ni comparta ninguna aguja usada. |
|
Paso 7 Después de la inyección
7.1 Compruebe que se ha administrado una inyección completa:
Si la ventana de información de la dosis muestra “0”, ha completado la dosis. Si la ventana de información de la dosis muestra un número mayor que “0”, la pluma precargada de GONAL-f está vacía. Usted no ha recibido la dosis prescrita completa y debe realizar elpaso 7.2 descrito a continuación. |
|
7.2 Complete una inyección parcial (sólo cuando sea necesario):
|
|
Paso 8 Conservación de la pluma precargada de GONAL-f
8.1 Vuelva a colocar el capuchón de la pluma sobre esta para evitar infecciones (Figura 34). 8.2 Guarde la pluma con el capuchón colocado en un lugar seguro y tal como se indica en el prospecto. 8.3 Cuando la pluma esté vacía, pregunte al profesional sanitario cómo desecharla. |
|
Noconserve la pluma con la aguja aún acoplada, ya que esto puede causar una infección.
Noreutilice la pluma precargada de GONAL-f si se ha caído, o si la pluma está agrietada o dañada, ya que esto puede causar lesiones.
Póngase en contacto con el profesional sanitario si tiene alguna pregunta.
Diario de tratamiento de la pluma precargada de GONAL-f
Fecha de la última revisión de estas instrucciones de uso: {MM/AAAA}.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a GONAL-F 150 UI/0,24 ML SOLUCION INYECTABLE EN PLUMA PRECARGADAForma farmacéutica: INYECTABLE, 150 UI/ 0,25 ml (11 microgramos/ 0,25 ml)Principio activo: Follitropin alfaFabricante: Gedeon Richter Plc.Requiere recetaForma farmacéutica: INYECTABLE, 150 UI/ 0,25 ml (11 microgramos/ 0,25 ml)Principio activo: Follitropin alfaFabricante: Gedeon Richter Plc.Requiere recetaForma farmacéutica: INYECTABLE, 150UI/0,25 ml (11 microgramos/ 0,25 ml)Principio activo: Follitropin alfaFabricante: Gedeon Richter Plc.Requiere receta
Médicos online para GONAL-F 150 UI/0,24 ML SOLUCION INYECTABLE EN PLUMA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de GONAL-F 150 UI/0,24 ML SOLUCION INYECTABLE EN PLUMA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes