GINE-CANESTEN 20 mg/g VAGINAL CREAM

How to use GINE-CANESTEN 20 mg/g VAGINAL CREAM
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Gine-Canestén 20 mg/g Vaginal Cream
Clotrimazole
Read this package leaflet carefully before you start using this medicine because it contains important information for you.
Follow exactly the administration instructions of the medicine contained in this package leaflet or as indicated by your doctor, pharmacist, or nurse.
- Keep this package leaflet, you may need to read it again.
- If you need advice or more information, consult your pharmacist.
- If you experience side effects, consult your doctor, pharmacist, or nurse, even if they are not listed in this package leaflet. See section 4.
- You should consult a doctor if your symptoms worsen or do not improve after 3 days.
Contents of the Package Leaflet:
- What is Gine-Canestén and what is it used for
- What you need to know before using Gine-Canestén
- How to use Gine-Canestén
- Possible side effects
- Storage of Gine-Canestén
Contents of the pack and additional information
1. What is Gine-Canestén and what is it used for
Clotrimazole is an antifungal (a medicine used to treat fungal infections).
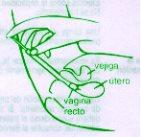
This medicine is indicated for the treatment of uncomplicated vulvovaginal candidiasis (vaginal infection caused by a fungus called Candida) (See section Warnings and precautions).
The main symptoms are itching, usually accompanied by an increase in vaginal discharge, pain, and redness of the vaginal and vulvar areas, burning, and a sensation of burning when urinating. These symptoms are not specific to vulvovaginal candidiasis. In case of doubt, consult your doctor.
2. What you need to know before using Gine-Canestén
Do not use Gine-Canestén
- If you are allergic (hypersensitive) to clotrimazole, imidazoles in general, or any of the other components of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor, pharmacist, or nurse before starting to use Gine-Canestén.
Do not ingest.
Before using this medicine, inform your doctor if you have problems with your immune system, for example, if you are being treated with oral corticosteroids or have HIV, AIDS, or diabetes.
You should consult a doctor if your symptoms worsen during treatment or persist after 3 days or if you notice an increase in vaginal discharge or changes in its appearance or odor, or if you experience vaginal bleeding.
In case of fever (38°C or higher), abdominal or back pain, lumbar pain, abundant watery vaginal discharge, and/or nausea, you should consult your doctor to rule out other types of illness.
Eye contact should be avoided, as it may cause irritation. If accidental contact with the eyes occurs, rinse with plenty of water and consult an ophthalmologist if necessary.
You should not use tampons, vaginal douches, spermicides, or other vaginal products while using this medicine.
Treatment should not be started during menstruation. Treatment should have ended before the start of menstruation.
In case of an allergic reaction during use, treatment should be discontinued and your doctor consulted immediately. Signs of a severe allergic reaction include swollen and itchy hives, swelling, sometimes on the face or mouth, causing difficulty breathing.
This medicine may reduce the effectiveness and safety of latex products, such as condoms and diaphragms. This effect is temporary and occurs only during treatment.
Sexual intercourse should be avoided in case of vaginal infection and while using this medicine to prevent the partner from becoming infected.
Use in children
Do not use in children under 12 years of age.
Use of Gine-Canestén with other medicines
Tell your doctor or pharmacist that you are taking, have recently taken, or might take any other medicines, especially if you are taking tacrolimus or sirolimus (medicines used in transplant patients)
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, or think you may be pregnant or are planning to become pregnant, consult your doctor or pharmacist before using this medicine.
Pregnancy
Clotrimazole may be used during pregnancy, but only under the supervision of a healthcare professional.
In case of treatment during the last 4 or 6 weeks of pregnancy, the use of the applicator is not recommended. Therefore, it is recommended to use vaginal tablets instead of vaginal cream, as they can be inserted directly with the finger, after careful hand washing.
Breastfeeding
Clotrimazole may be used during breastfeeding.
Driving and using machines
The influence of Gine-Canestén on the ability to drive and use machines is negligible.
Gine-Canestén contains cetyl alcohol and benzyl alcohol
This medicine may cause local skin reactions (such as contact dermatitis) because it contains cetyl alcohol.
This medicine contains 20 mg of benzyl alcohol in each gram of cream.
Benzyl alcohol may cause allergic reactions.
Benzyl alcohol may cause moderate local irritation.
3. How to use Gine-Canestén
Follow exactly the administration instructions of this medicine contained in this package leaflet or as indicated by your doctor, pharmacist, or nurse. In case of doubt, ask your doctor, pharmacist, or nurse.
The recommended dose is:
Adults and adolescents over 12 years of age
This medicine is administered vaginally.
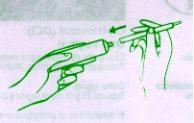
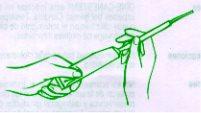
It is usually sufficient to apply one charge of the vaginal applicator (approximately 5 g) once a day, preferably at night before bedtime, for 3 consecutive days. The cream should be inserted deeply into the vagina (see instructions for use of the applicator), with the patient lying on her back and with her legs slightly bent.
If symptoms persist after completing treatment or recur after 2 months, or if you have problems with your immune system, HIV, AIDS, or diabetes, you should consult your doctor.
Use in children
Do not use in children under 12 years of age.
If you use more Gine-Canestén than you should
Accidental ingestion may cause gastrointestinal discomfort and/or vomiting. Accidental administration in the eyes may cause burning and eye irritation without gravity.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service. Phone (91) 562 04 20 indicating the medicine and the amount ingested.
If you forget to use Gine-Canestén
In case of forgetting a dose, wait for the next one. Do not apply a double dose to make up for the forgotten dose.
If you stop treatment with Gine-Canestén
Continue using Gine-Canestén until you finish the treatment, even if you feel better. You need to complete the treatment to cure the infection. If you stop treatment, the fungus may not have disappeared.
If you have any other questions about the use of this medicine, ask your doctor.
4. Possible side effects
Like all medicines, Gine-Canestén can cause side effects, although not everybody gets them.
Adverse reactions with frequency not known (cannot be estimated from the available data) are:
Immune system disorders:
Angioedema (swelling under the skin), allergic reaction, hypersensitivity.
Vascular disorders:
Syncope (sudden loss of consciousness, fainting), hypotension (low blood pressure).
Respiratory, thoracic, and mediastinal disorders:
Breathing difficulties.
Gastrointestinal disorders:
Abdominal pain, nausea.
Skin and subcutaneous tissue disorders:
Rash, urticaria (elevated red itchy patches).
Reproductive system and breast disorders:
Vaginal scaling, vaginal discharge, vulvovaginal itching, vulvovaginal erythema, burning sensation in the genital area, vulvovaginal discomfort, vulvovaginal pain, and vaginal bleeding
General disorders and administration site conditions:
Irritation at the application site, edema, pain.
These symptoms usually do not determine the suppression of treatment and are more frequent during the first days of treatment.
Reporting of side effects:
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. You can also report them directly through the Spanish Medicines and Health Products Agency: http://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Gine-Canestén
No special storage conditions are required.
Keep this medicine out of the sight and reach of children.
Do not use Gine-Canestén after the expiry date stated on the packaging, after the abbreviation EXP. The expiry date is the last day of the month indicated.
Medicines should not be disposed of via wastewater or household waste. Place the packaging and any unused medicine in the pharmacy's SIGRE collection point. If in doubt, consult your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Contents of the pack and additional information
Composition of Gine-Canestén
- The active ingredient is clotrimazole. Each gram of cream contains 20 mg of clotrimazole.
- The other ingredients (excipients) are: sorbitan stearate (E491), polysorbate 60 (E435), cetyl palmitate, cetyl alcohol, octyldodecanol, benzyl alcohol (E1519), purified water.
Appearance of the product and contents of the pack:
This medicine is a white cream. It is presented in a cardboard box containing an aluminum tube with a plastic cap, containing 20 g of cream, and 3 disposable vaginal applicators.
Marketing authorization holder
BAYER HISPANIA, S.L.
Av. Baix Llobregat, 3 – 5
08970 Sant Joan Despí (Barcelona)
Spain
Manufacturer
GP Grenzach Produktions GmbH
Emil-Barell-Str. 7
79639 Grenzach-Wyhlen
Germany
Date of last revision of this package leaflet: February 2022
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
INSTRUCTIONS FOR USE OF THE APPLICATOR
|
|
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to GINE-CANESTEN 20 mg/g VAGINAL CREAMDosage form: VAGINAL SUPPOSITORY/CAPSULE/TABLET, 100 mg clotrimazoleActive substance: clotrimazoleManufacturer: Bayer Hispania S.L.Prescription requiredDosage form: VAGINAL SEMISOLID, 2% clotrimazole / 100 gActive substance: clotrimazoleManufacturer: Bayer Hispania S.L.Prescription requiredDosage form: VAGINAL SUPPOSITORY/CAPSULE/TABLET, 500 mg clotrimazoleActive substance: clotrimazoleManufacturer: Bayer Hispania S.L.Prescription required
Online doctors for GINE-CANESTEN 20 mg/g VAGINAL CREAM
Discuss questions about GINE-CANESTEN 20 mg/g VAGINAL CREAM, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions