
GELISTROL 50 micrograms/g VAGINAL GEL

How to use GELISTROL 50 micrograms/g VAGINAL GEL
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
PACKAGE LEAFLET: INFORMATION FOR THE USER
Gelistrol50 micrograms/g vaginal gel
Estriol
Read all of this leaflet carefully before you start usingthismedicinebecause it contains important information for you
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information:
- What is Gelistrol and what is it used for
- What you need to know before you use Gelistrol
- How to use Gelistrol
- Possible side effects
- Storing Gelistrol
- Contents of the pack and further information
1. What is Gelistrol and what is it used for
Gelistrol belongs to a group of medicines called local vaginal hormone replacement therapy (HRT).
It is used to relieve symptoms of the menopause in the vagina, such as dryness or irritation. In medical terms, this is known as "vaginal atrophy". This is caused by a decrease in estrogen levels in your body and occurs naturally after menopause.
Gelistrol works by replacing the estrogen that is normally produced by the ovaries. It is inserted into the vagina, so that the hormone is released only where it is needed, and can thus relieve the discomfort at the vaginal level.
2. What you need to know before you use Gelistrol
Medical history and check-ups
The use of hormone replacement therapy involves risks that need to be considered when starting or continuing treatment.
Before you start (or restart) hormone replacement therapy, your doctor will ask you about your medical history and that of your family. Your doctor may decide to perform a physical examination, which may include a breast examination and/or an internal examination, if necessary.
Once you have started taking Gelistrol, you should see your doctor for regular check-ups (at least once a year). During these check-ups, discuss the benefits and risks of continuing Gelistrol with your doctor.
Have regular mammograms as recommended by your doctor.
Do not use Gelistrol
- If any of the following conditions apply to you, or if you are unsure about any of the following, consult your doctor before using Gelistrol: if you have, have had, or suspect you may have breast cancer,
- if you have or suspect you may have estrogen-sensitive cancer, such as endometrial cancer (lining of the uterus),
- if you have unexplained vaginal bleeding,
- if you have untreated endometrial hyperplasia (abnormal growth of the lining of the uterus),
- if you have or have had blood clots in the veins of the legs (deep vein thrombosis) or lungs (pulmonary embolism),
- If you have a blood clotting disorder (such as protein C, protein S, or antithrombin deficiency),
- if you have or have recently had diseases caused by blood clots in the arteries, such as a stroke, angina, or heart attack,
- if you have or have had a severe liver disease and your liver function tests have not returned to normal,
- if you have porphyria (a rare inherited metabolic disorder),
- if you are allergic(hypersensitive) to estriol or any of the other components of Gelistrol (listed in section 6 "Further information").
If any of the above conditions occur for the first time while taking Gelistrol, stop taking it immediately and consult your doctor immediately.
Warnings and precautions
This medicine is administered by inserting an applicator into the vagina. This may cause discomfort or pain in women with severe vaginal atrophy (narrowing or inflammation of the vaginal walls).
Please inform your doctor if you have or have had any of the following diseases/disorders, which can rarely recur or worsen during treatment with Gelistrol. If so, you should see your doctor more frequently for medical check-ups:
- a very high level of fat in the blood (triglycerides)
- growth of tissue that lines the inside of the uterus outside the uterus (endometriosis) or a history of excessive growth of tissue that lines the uterus (endometrial hyperplasia)
- fibroids inside the uterus
- high blood pressure
- diabetes
- gallstones
- migraine or severe headache
- a rare immune system disease called systemic lupus erythematosus (SLE)
- epilepsy (seizures)
- asthma
- a disease that affects the eardrum and hearing (otosclerosis)
- fluid retention due to heart or kidney problems
- increased risk of developing blood clots (see "blood clots in a vein (thrombosis)"),
- increased risk of developing an estrogen-sensitive cancer (e.g., if your mother, sister, or grandmother had breast cancer)
- a liver disorder, such as a benign liver tumor
- hereditary and acquired angioedema
Reasons to see your doctor immediately
- jaundice (yellowing of the whites of the eyes and skin) or liver disorders
- sudden increase in blood pressure
- pregnancy
- painful swelling and redness of the legs
- sudden chest pain
- difficulty breathing.
For more information, see "blood clots in a vein (thrombosis)".
If any of the above conditions occur, your doctor may need to stop treatment and offer you an alternative.
Note: Gelistrol is not a contraceptive. If it has been less than 12 months since your last period or if you are under 50 years old, you may need to use additional contraceptive methods to prevent pregnancy. Talk to your doctor for advice.
Hormone replacement therapy and cancer
Excessive thickening of the tissue that lines the inside of the uterus (endometrial hyperplasia) and cancer of the tissue that lines the inside of the uterus.
Taking estrogen-only HRT tablets for a long time may increase the risk of cancer of the uterine lining (the endometrium).
It is not clear if there is a similar risk with Gelistrol when used for repeated or long-term treatment (more than one year). However, it has been shown that the absorption of Gelistrol into the bloodstream is very low; therefore, the addition of a progestogen is not necessary.
If you bleed or spot, it is usually nothing to worry about, but you should schedule an appointment to see your doctor. It could be a sign that the endometrium has become thicker.
The following risks apply to hormone replacement therapy (HRT) medicines that circulate in the blood. Now, Gelistrol is used for local treatment in the vagina and absorption into the blood is very small. It is less likely that the disorders mentioned below will worsen or recur during treatment with Gelistrol, but you should see your doctor if you are concerned.
Treatment with medicines containing higher doses of estrogen that can increase estrogen levels in the blood (such as tablets or patches) increases the risk of abnormal growth of the endometrium (endometrial hyperplasia), certain types of cancer such as breast and endometrial cancer, and blood clots in the veins.
Breast cancer
Available data indicate that using Gelistrol does not increase the risk of breast cancer in women who have not had breast cancer in the past. It is not known if Gelistrol can be used safely in women who have had breast cancer in the past.
Check your breasts regularly. See your doctor if you notice any changes such as:
- dimpling of the skin
- changes in the nipple
- any lump that you can see or feel
In addition, you are advised to participate in breast screening programs when they are offered.
Ovarian cancer
Ovarian cancer is rare: much rarer than breast cancer. The use of estrogen-only HRT has been associated with a slightly increased risk of ovarian cancer.
The risk of ovarian cancer varies with age. For example, in women aged 50-54 who are not taking HRT, about 2 women in 2000 will be diagnosed with ovarian cancer over a 5-year period. For women who have been taking HRT for 5 years, there will be about 3 cases per 2000 users (i.e., about 1 extra case).
Effect of hormone replacement therapy on the heart and circulation
Blood clots in a vein
The risk of blood clots in the veins is about 1.3 to 3 times higher in women using hormone replacement therapy than in women not using it, especially during the first year of treatment.
Blood clots can be serious, and if one travels to the lungs, it can cause chest pain, shortness of breath, fainting, or even death.
The likelihood of having a blood clot in the veins increases with age and if you are affected by any of the following. Inform your doctor if any of these apply to you:
- you are unable to walk for a long time due to major surgery, injury, or illness,
- you are significantly overweight (BMI >30 kg/m2),
- you have a blood clotting disorder that requires long-term treatment with a medicine to prevent blood clots,
- if a close relative has had a blood clot in the leg, lung, or other organ,
- you have systemic lupus erythematosus (SLE),
- you have cancer.
Comparison
An average of 4 to 7 out of 1,000 women in their 50s who are not taking hormone replacement therapy may have a blood clot in a vein over a 5-year period. In women in their 50s who have taken estrogen-only hormone replacement therapy for more than 5 years, there will be 5 to 8 cases per 1,000 users (i.e., 1 extra case).
Heart disease (heart attack)
Women taking only estrogen do not have an increased risk of developing heart disease.
Stroke
The risk of having a stroke is about 1.5 times higher in women using hormone replacement therapy than in women not using it. The number of extra stroke cases due to hormone replacement therapy increases with age.
Comparison
An average of 8 out of 1,000 women in their 50s who are not taking hormone replacement therapy may have a stroke over a 5-year period. In women in their 50s taking hormone replacement therapy for more than 5 years, there will be 11 cases per 1,000 users (i.e., 3 extra cases).
Using Gelistrol with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines, including those obtained without a prescription, herbal medicines, or other natural products.
Since Gelistrol contains a very low dose of estriol and is for local treatment, it is not expected to affect or be affected by the use of other medicines. However, interactions with other local vaginal treatments should be considered.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Do not use Gelistrol if you are pregnant.
If you become pregnant during treatment, inform your doctor immediately and do not use Gelistrol.
Do not use Gelistrol during breastfeeding.
Driving and using machines
Gelistrol does not affect the ability to drive and use machines.
Gelistrol contains
Methyl parahydroxybenzoate, sodium salt (E 219) and propyl parahydroxybenzoate, sodium salt (E 217).
Do not use this medicine if you are allergic to any of its components.
3. How to use Gelistrol
Follow the instructions for using Gelistrol exactly as indicated by your doctor or pharmacist. If you are unsure, consult your doctor or pharmacist again.
The recommended dose during the first three weeks of treatment is one application per day, preferably before bedtime. After 3 weeks of use, your symptoms should have decreased and the dose should be reduced. You may only need to apply it twice a week.
Use the applicator to insert the gel into the vagina (recommended before bedtime).
Your doctor will try to prescribe the lowest dose to treat your symptoms for the shortest necessary time. Consult your doctor if you think this dose is too strong or not strong enough.
The following instructions explain how to use the gel.
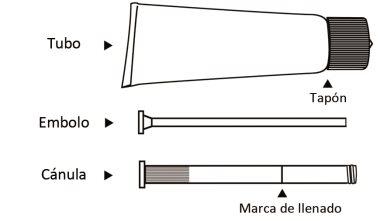
In the following drawing, you can see all the components of the tube and applicator, consisting of the cannula and plunger.
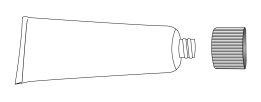
- Remove the cap from the tube, invert the cap, and use the pointed tip to break the seal on the neck of the tube. Do not use if the seal is broken.
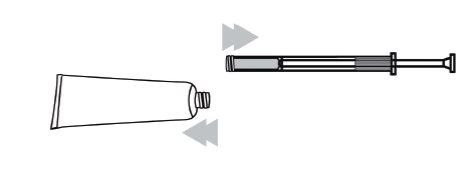
- Remove the cannula and plunger. Insert the white plunger along the cannula. Screw the cannula onto the end of the tube.

- Squeeze the tube to fill the applicator with the gel up to the fill mark. The applicator will stop at the mark.

- Remove the cannula from the tube and put the cap back on the tube.
- To apply the gel, lie down, insert the tip of the applicator deeply into the vagina, and slowly push the plunger down.
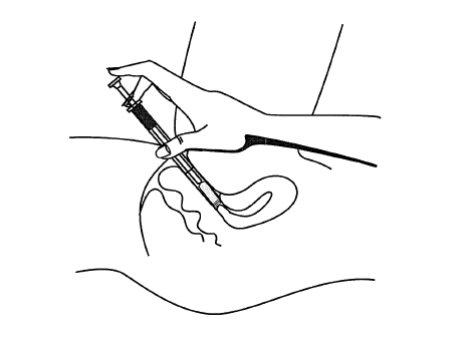
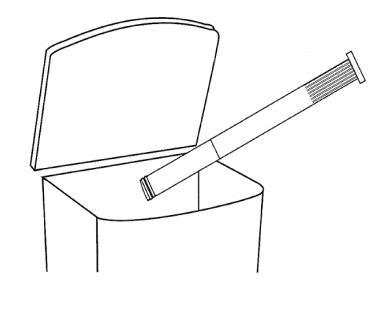
- After use
- 10g tube – 1 Blister with 10 disposable tubes (cannulas) and 1 reusable plunger
- 30g tube – 3 Blisters with 30 disposable tubes (cannulas) and 1 reusable plunger
Remove the plunger from the cannula, discard the cannula, and clean the plunger well with warm water to use it again for the next application.
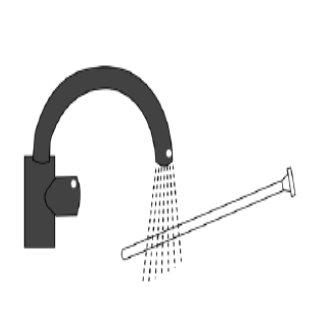
- 10g tube – 1 Bag with 1 reusable tube (cannula) and 1 reusable plunger
- 30g tube – 1 Bag with 1 reusable tube (cannula) and 1 reusable plunger
Remove the plunger from the cannula and clean the cannula and plunger well with warm water to use them again for the next application.
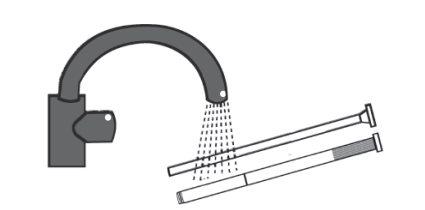
If you use more Gelistrol than you should
If you apply too much gel or someone has accidentally ingested some gel, do not worry. You may feel unwell, and in some women, vaginal bleeding may occur after a few days.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount ingested.
If you forget to use Gelistrol
Do not use a double dose to make up for forgotten doses.
Apply the forgotten dose when you remember, unless more than 12 hours have passed. In this case, simply skip the forgotten dose.
If you have any other questions about using this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like any other medication, this medication may produce adverse effects, although not all people suffer from them.
Inform your doctor immediatelyif any of the circumstances described in the section "What you need to know before starting to use Gelistrol" occur, such as vaginal bleeding. Your doctor may need to interrupt treatment and offer you an alternative.
At the beginning of treatment, irritation or itching in the vagina may occur. In most patients, these adverse effects disappear with continued use. Inform your doctor in case of vaginal bleeding or if any of the following adverse effects worsen or continue.
Frequent Adverse Effects (may affect up to 1 in 10 people):
- Itching, irritation inside or around the vagina.
Uncommon Adverse Effects (may affect up to 1 in 100 patients):
- Lower abdominal pain,
- skin irritation,
- vaginal rash,
- headache,
- candidiasis (vaginal infection).
The following diseases are reported more frequently in women using systemic hormone replacement therapy (HRT) compared to women not using HRT. These risks occur less frequently in treatments administered via the vaginal route, such as Gelistrol:
- Blood clots in the veins of the legs or lungs (venous thromboembolism).
- Ovarian cancer.
- Stroke.
- Probable memory loss if HRT is started after the age of 65.
For more information about these adverse effects, see Section 2.
The following adverse effects have been reported in association with other hormone replacement therapies.
- Bile duct diseases,
- various skin disorders:
- skin pigmentation, especially on the face or neck, known as "pregnancy spots" (chloasma),
- painful and reddish nodules on the skin (erythema nodosum),
- skin rash with reddish lesions or papules in a target shape (erythema multiforme).
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor, pharmacist, or nurse, even if it is a possible adverse effect that is not listed in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Gelistrol
Keep out of sight and reach of children.
Do not use this medication after the expiration date stated on the packaging.
The expiration date is the last day of the month indicated.
Store below 25°C.
Medicines should not be disposed of through wastewater or household waste. Deposit the packaging and any unused medicines in the pharmacy's SIGRE collection point. In case of doubt, ask your pharmacist how to dispose of the packaging and any unused medicines. This will help protect the environment.
6. Package Contents and Additional Information
Composition ofGelistrol
The active ingredient is estriol.
A full applicator, up to the mark, corresponds to a dose of 1 g of vaginal gel. Each gram of gel contains 50 micrograms of estriol.
The other components are: Glycerol (E 422), methyl parahydroxybenzoate, sodium salt (E 219), propyl parahydroxybenzoate, sodium salt (E 217), polycarbophil, carbomer, sodium hydroxide, hydrochloric acid, purified water.
Appearance of Gelistrol and Package Contents
This medication is a colorless, transparent, or slightly translucent vaginal gel, which contains a 10g or 30g aluminum tube.
- Presentation 10g - Blister pack with 10 disposable tubes (canulas) and a reusable plunger.
Cardboard box with a 10g tube of Gelistrol and a blister pack with 10 disposable tubes (canulas) marked with a filling mark and a reusable plunger.
- Presentation 10g - Bag with 1 reusable tube (canula) and a reusable plunger.
Cardboard box with a 10g tube of Gelistrol and a bag with 1 reusable tube (canula) marked with a filling mark and a reusable plunger.
- Presentation 30g - 3 Blister packs with 10 disposable tubes (canulas) and a reusable plunger each.
Cardboard box with a 30g tube of Gelistrol and three blister packs, each with 10 disposable tubes (canulas) marked with a filling mark and a reusable plunger.
- Presentation 30g - Bag with 1 reusable tube (canula) and a reusable plunger.
Cardboard box with a 30g tube of Gelistrol and a bag with 1 reusable tube (canula) marked with a filling mark and a reusable plunger.
Only some package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder:
ITALFARMACO, S.A.
San Rafael, 3 - 28108 Alcobendas (Madrid)
Manufacturer Responsible for Release:
ITALFARMACO, S.A.
San Rafael, 3 - 28108 Alcobendas (Madrid), SPAIN
Local Representative
Laboratorios EFFIK S.A.
San Rafael, 3 – 28108 Alcobendas (Madrid), SPAIN
Tel: + 34 91 3585273
This medication is authorized in the member states of the European Economic Area under the following names:
- Spain: Gelistrol 50 micrograms/g vaginal gel
- Sweden: Gelistrol 50 mikrogram/g vaginal gel
- France: Gelistrol 50 microgrammes/g gel vaginal
- Italy: Gelistrol 50 microgrammi/g gel vaginale
- Portugal: Gelistrol 50 microgramas/g gel vaginal
- Greece: Gelistrol μικρογραμμ?ρια/g κολπικ? γ?λη
Date of the Last Revision of this Prospectus:March 2022
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to GELISTROL 50 micrograms/g VAGINAL GELDosage form: VAGINAL SEMISOLID, 50 micrograms/gActive substance: estriolManufacturer: Italfarmaco S.A.Prescription requiredDosage form: VAGINAL SUPPOSITORY/CAPSULE/TABLET, 0.03 mgActive substance: estriolManufacturer: Kern Pharma S.L.Prescription requiredDosage form: VAGINAL SUPPOSITORY/CAPSULE/TABLET, 0.5 mg estriolActive substance: estriolManufacturer: Aspen Pharma Trading LimitedPrescription required
Online doctors for GELISTROL 50 micrograms/g VAGINAL GEL
Discuss questions about GELISTROL 50 micrograms/g VAGINAL GEL, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











