
FORTASEC FLAS 2 mg ORAL LYOPHILIZED

How to use FORTASEC FLAS 2 mg ORAL LYOPHILIZED
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What is Fortasec Flas 2 mg oral lyophilized and what is it used for
- What you need to know before taking Fortasec Flas 2 mg oral lyophilized
- How to take Fortasec Flas 2 mg oral lyophilized
- Possible side effects
- Storage of Fortasec Flas 2 mg oral lyophilized
- Package contents and additional information
Introduction
Fortasec Flas 2 mg oral lyophilized
Loperamide hydrochloride
Read the entire leaflet carefully before starting to take this medication, as it contains important information for you
Follow the administration instructions for the medication contained in this leaflet or as indicated by your doctor or pharmacist exactly.
- Keep this leaflet as you may need to read it again.
- If you need advice or more information, consult your pharmacist.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet.
- You should consult a doctor if it worsens or does not improve after 2 days.
Contents of the leaflet:
- What Fortasec Flas 2 mg oral lyophilizedis and what it is used for.
- What you need to know before taking Fortasec Flas 2 mg oral lyophilized.
- How to take Fortasec Flas 2 mg oral lyophilized.
- Possible side effects.
- Storage of Fortasec Flas 2 mg oral lyophilized.
- Package contents and additional information.
1. What is Fortasec Flas 2 mg oral lyophilized and what is it used for
It is an antidiarrheal medication that reduces intestinal movements and secretions, resulting in a decrease in liquid stools.
It is indicated for the symptomatic treatment of acute non-specific diarrhea (occasional) in adults and children over 12 years of age.
You should consult a doctor if it worsens or does not improve after 2 days.
2. What you need to know before taking Fortasec Flas 2 mg oral lyophilized
Do not take Fortasec Flas 2 mg oral lyophilized
- Children under 12 years of age
- If you are allergic (hypersensitive) to loperamide hydrochloride or any of the other components of this medication (listed in section 6)
- If you have a high fever (above 38°C) or blood in your stool
- If you suffer from ulcerative colitis (inflammation of the intestine)
- If you have severe diarrhea after taking antibiotics.
- If you have diarrhea due to an infection caused by organisms such as Salmonella, Shigella, and Campylobacter
- Treatment should be suspended immediately and a doctor consulted if constipation, abdominal distension (swelling), or ileus (absence of intestinal movements) occurs.
Warnings and precautions
Consult your doctor or pharmacist before starting to take Fortasec Flas 2 mg.
If you have severe diarrhea, your body loses more fluids, sugars, and salts than usual, and you will need to replenish fluids by drinking more than usual. Ask your pharmacist about preparations to replenish sugars and salts.
- The treatment with this medication is only symptomatic, so the diarrhea should be treated from its cause.
- If you have AIDS and your stomach swells, stop taking this medication immediately and consult your doctor
- If you have liver disease, consult your doctor before taking this medication, as it could produce toxicity in the central nervous system.
- If you are taking any narcotic analgesic, consult your doctor or pharmacist before taking this medication, as it may interact with them.
If you do not observe improvement within 48 hours or if fever, constipation, or other symptoms appear, discontinue treatment and consult your doctor.
Patient with diarrhea should drink plenty of fluids to avoid dehydration, which is manifested by dry mouth, excessive thirst, decreased urine output, wrinkled skin, dizziness, and drowsiness. This is especially important in children and the elderly.
Do not take this medication for a use other than indicated (see section 1) and never take more than the recommended amount (see section 3). Serious heart problems (whose symptoms include rapid or irregular heartbeats) have been reported in patients who have taken an excessive amount of loperamide, the active ingredient of Fortasec Flas.
Children and adolescents
Do not use in children or adolescents under 12 years of age.
Use of Fortasec Flas with other medications
Tell your doctor or pharmacist that you are using or have recently used or may need to use any other medication.
- It is considered especially important if you are taking medications that contain
- Ritonavir, saquinavir (used to treat HIV).
- Quinidine (used to treat abnormal heart rhythms).
- Desmopressin oral (used to treat central diabetes insipidus and nocturnal urinary incontinence in children).
- Itraconazole or ketoconazole (used to treat fungal infections).
- Gemfibrozil (used to lower cholesterol).
- St. John's Wort (used to improve mood).
- Valerian (used to treat mild anxiety).
- Opioid analgesics (used to treat very intense pain) as it may increase the risk of severe constipation and central nervous system depression (e.g., drowsiness or decreased consciousness).
- Broad-spectrum antibiotics as Fortasec may worsen or prolong antibiotic-induced diarrhea and increase loperamide levels in the blood.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before taking this medication.
Pregnant women should not take Fortasec Flasunless prescribed by a doctor, as there is no experience with its use in pregnant women.
Do not take Fortasec Flasif you are breastfeeding. Small amounts of the medication may pass into breast milk.
Driving and using machines
You can drive and use machines unless you feel drowsy, dizzy, or somnolent. You cannot drive in those situations.
Warnings about excipients
Fortasec Flas contains aspartame (E-951)
This medication contains 0.750mg of aspartame in each lyophilized oral form.
Aspartame contains a source of phenylalanine that may be harmful in case of phenylketonuria (PKU), a rare genetic disease in which phenylalanine accumulates because the body is unable to eliminate it properly.
Fortasec Flas contains sodium:
This medication contains less than 23 mg of sodium (1mmol) per lyophilized oral form; that is, it is essentially "sodium-free".
Fortasec Flas contains propylene glycol (E-1520)
This medication contains 0.00003 mg of propylene glycol (E-1520) in each lyophilized oral form.
Fortasec Flas contains benzyl alcohol
This medication contains 0.00066 mg of benzyl alcohol in each lyophilized oral form. Benzyl alcohol may cause allergic reactions. Consult your doctor or pharmacist if you are pregnant or breastfeeding. This is because large amounts of benzyl alcohol can accumulate in your body and cause adverse effects (metabolic acidosis).
Consult your doctor or pharmacist if you have liver or kidney disease. This is because large amounts of benzyl alcohol can accumulate in the body and cause adverse effects (metabolic acidosis).
Fortasec Flas contains corn maltodextrin
This medication contains corn maltodextrin that contains glucose. If your doctor has told you that you have an intolerance to certain sugars, consult with them before taking this medication.
Fortasec Flas contains traces of sulfites
This medication may cause severe allergic reactions and bronchospasm because the peppermint flavor contains traces of sulfites.
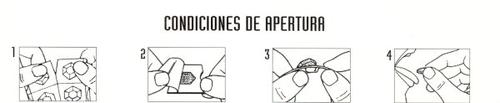
3. How to take Fortasec Flas 2 mg oral lyophilized
Follow the administration instructions for the medication contained in this leaflet or as indicated by your doctor or pharmacist exactly. In case of doubt, ask your doctor or pharmacist.
The recommended dose is:
Adults
2 lyophilized oral forms (4 mg) as an initial dose followed by 1 lyophilized oral form (2 mg) after each diarrheal stool.
The maximum dose for adults is 8 lyophilized oral forms (16 mg) per day.
Childrenover 12 years
1 lyophilized oral form (2 mg) as an initial dose followed by 1 lyophilized oral form (2 mg) after each diarrheal stool.
In children, the maximum daily dose should be related to body weight:
Child's weight | Maximum number oflyophilized oral formsper day |
From 27 kg | Maximum 4 lyophilized oral forms |
From 34 kg | Maximum 5 lyophilized oral forms |
From 40 kg | Maximum 6 lyophilized oral forms |
From 47 kg | Maximum 7 lyophilized oral forms |
Patient with liver disease:Should consult a doctor.
Advanced age and patients with kidney disease
Follow the same instructions as for adults and children over 12 years of age
Use in children and adolescents
Children under 12 years of age should not take this medication. It is contraindicated.

If you take more Fortasec Flas 2 mg oral lyophilized than you should
Taking too many lyophilized oral forms can cause abnormal coordination, stupor, pupil constriction, increased muscle tension, difficulty breathing (children are more sensitive to these effects than adults), and reduced urine output.
In case of overdose or accidental ingestion, consult your doctor or pharmacist, or call the Toxicology Information Service, Phone 91 562 04 20 (indicating the medication and the amount ingested).
If you forget to take Fortasec
Do not take a double dose to make up for forgotten doses.
If you have any other doubts about the use of this medication, ask your doctor or pharmacist
4. Possible side effects
Like all medications, Fortasec Flascan cause side effects, although not everyone will experience them.
Frequency not known (cannot be estimated from available data)
Upper abdominal pain, abdominal pain radiating to the back, abdominal tenderness, fever, rapid pulse, nausea, vomiting, which can be symptoms of pancreatitis (acute pancreatitis).
If you experience any of these symptoms, stop using the medication and seek immediate medical attention.
Very rare adverse reactions(may affect up to 1 in 10,000 patients):
- Swelling of the face, lips, tongue, or throat that can cause difficulty swallowing or breathing (angioedema)
- Allergic reactions (including anaphylactic shock)
- Glossodynia
- Fatigue
- Decreased urine output
- Loss or decreased consciousness
- Abnormal coordination
- Increased muscle tension (hypertonia)
- Stupor
- Pupil constriction (miosis)
- Loss of intestinal mobility (ileus, including paralytic ileus)
- Pruritus or urticaria
- Somnolence
- Abnormal colon dilation (megacolon)
- Bullous eruption (including Stevens-Johnson syndrome, toxic epidermal necrolysis, and erythema multiforme)
Reporting side effects
If you experience any type of side effect, consult your doctor or pharmacist, even if it is a possible side effect not listed in this leaflet. You can also report them directly through the Spanish Medication Surveillance System for Human Use: https://www.notificaram.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Fortasec Flas 2 mg oral lyophilized
This medication does not require any special storage temperature. Keep in the original packaging to protect it from moisture.
Keep out of sight and reach of children.
Do not use this medication after the expiration date shown on the packaging after "Expiration Date". The expiration date is the last day of the month indicated.
Medications should not be thrown away in drains or trash. Deposit the packaging and medications you no longer need in the Sigre Collection Point at the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and medications you no longer need. This way, you will help protect the environment.
6. Package contents and additional information
Composition of Fortasec Flas 2 mg oral lyophilized
The active ingredient is loperamide 1.86 mg provided by 2 mg of loperamide hydrochloride.
The other components (excipients) are: gelatin, mannitol (E-421), aspartame (E-951), peppermint flavor (contains corn maltodextrin (contains glucose), propylene glycol (E-1520), benzyl alcohol, and traces of sulfites), and sodium hydrogen carbonate.
Appearance of the product and package contents
Fortasec Flas are lyophilized oral forms.
It is available in packages of 6 and 12 lyophilized oral forms.
Marketing authorization holder and manufacturer:
Marketing authorization holder
JNTL Consumer Health (Spain), S.L.
C/ Vía de los Poblados 1, Edificio E, planta 3
28033-Madrid
Spain
Manufacturer
JANSSEN-CILAG S.P.A.
Via C. Janssen
04010 Borgo S.Michele
Latina (Italy)
or
JNTL Consumer Health (France) S.A.S.
Domaine de Maigremont
Val de Reuil
- France
Date of the last revision of this leaflet: March 2022
Detailed and updated information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FORTASEC FLAS 2 mg ORAL LYOPHILIZEDDosage form: TABLET, 2 mgActive substance: loperamideManufacturer: Uxa Farma S.A.Prescription not requiredDosage form: CAPSULE, 2 mg loperamide hydrochlorideActive substance: loperamideManufacturer: Laboratorios Cinfa S.A.Prescription not requiredDosage form: ORALLY DISINTEGRATING TABLET/LIOTAB, 2 mgActive substance: loperamideManufacturer: Laboratorios Cinfa S.A.Prescription not required
Online doctors for FORTASEC FLAS 2 mg ORAL LYOPHILIZED
Discuss questions about FORTASEC FLAS 2 mg ORAL LYOPHILIZED, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















