
FLUCONAZOLE AUROVITAS 200 mg HARD CAPSULES

How to use FLUCONAZOLE AUROVITAS 200 mg HARD CAPSULES
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Fluconazole Aurovitas 50 mg hard capsules EFG
Fluconazole Aurovitas 100 mg hard capsules EFG
Fluconazole Aurovitas 150 mg hard capsules EFG
Fluconazole Aurovitas 200 mg hard capsules EFG
fluconazole
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Fluconazole Aurovitas and what is it used for
- What you need to know before you take Fluconazole Aurovitas
- How to take Fluconazole Aurovitas
- Possible side effects
- Storing Fluconazole Aurovitas
- Contents of the pack and other information
1. What is Fluconazole Aurovitas and what is it used for
This medicine is one of a group of medicines called “antifungals”. The active substance is fluconazole.
This medicine is used to treat infections caused by fungi, and it can also be used to prevent the appearance of candidiasis. The most common cause of fungal infections is a yeast called Candida.
Adults
Your doctor may prescribe this medicine to treat the following types of fungal infections:
- cryptococcal meningitis – a fungal infection in the brain,
- coccidioidomycosis – a disease of the bronchopulmonary system,
- Candidainfections localized in the bloodstream, in body organs (e.g. heart, lungs) or in the urinary tract,
- mucosal candidiasis – an infection that affects the lining of the mouth, throat or associated with dental prostheses,
- genital candidiasis – an infection of the vagina or penis,
- skin infections – e.g. athlete's foot, ringworm, inguinal ringworm, nail infection.
It may also be prescribed for you to:
- prevent the recurrence of cryptococcal meningitis,
- prevent the recurrence of mucosal infections,
- reduce the repeated appearance of vaginal infections,
- prevent the spread of Candidainfections (if your immune system is weak and not working properly).
Children and adolescents (0 to 17 years)
Your doctor may prescribe this medicine to treat the following types of fungal infections:
- mucosal candidiasis – an infection that affects the lining of the mouth or throat,
- Candidainfections localized in the bloodstream, in body organs (e.g. heart, lungs) or in the urinary tract,
- cryptococcal meningitis – a fungal infection in the brain.
It may also be prescribed for you to:
- prevent the spread of Candidainfections (if your immune system is weak and not working properly),
- prevent the recurrence of cryptococcal meningitis.
2. What you need to know before you take Fluconazole Aurovitas
Do not take Fluconazole Aurovitas:
- if you are allergic to fluconazole, to other medicines you have taken to treat fungal infections or to any of the other ingredients of this medicine (listed in section 6). The symptoms can include itching, skin redness or difficulty breathing,
- if you are taking astemizole, terfenadine (antihistamine medicines for treating allergies),
- if you are taking cisapride (used for stomach upset),
- if you are taking pimozide (used for treating mental illnesses),
- if you are taking quinidine (used for treating heart arrhythmias),
- if you are taking erythromycin (an antibiotic for treating infections).
Warnings and precautions
Consult your doctor or pharmacist before starting to take this medicine.
- if you have liver or kidney problems,
- if you have a heart condition, including heart rhythm problems,
- if you have abnormal levels of potassium, calcium or magnesium in the blood,
- if severe skin reactions occur (itching, skin redness or difficulty breathing),
- if you develop signs of adrenal insufficiency, where the adrenal glands do not produce adequate amounts of certain steroid hormones such as cortisol (chronic or long-term, fatigue, muscle weakness, loss of appetite, weight loss, abdominal pain),
- if you have ever had a severe skin rash or skin peeling and/or blisters and/or sores in the mouth after taking fluconazole.
Severe skin reactions, including drug reaction with eosinophilia and systemic symptoms (DRESS), have been reported in relation to fluconazole treatment. Stop taking fluconazole and seek immediate medical attention if you notice any of the symptoms related to these severe skin reactions described in section 4.
Talk to your doctor or pharmacist if the fungal infection does not improve, it may be necessary to use an alternative antifungal treatment.
Other medicines and Fluconazole Aurovitas
Immediatelyinform your doctor if you are taking astemizole, terfenadine (an antihistamine for treating allergies), cisapride (used for stomach upset), pimozide (used for treating mental illnesses), quinidine (used for treating heart arrhythmias) or erythromycin (an antibiotic for treating infections), as these medicines should not be taken with this medicine (see section: “Do not take Fluconazole Aurovitas”).
There are some medicines that may interact with this medicine. Make sure your doctor knows if you are taking any of the following medicines, as it may be necessary to adjust the dose or monitor to check that the medicines are still having the desired effect:
- rifampicin or rifabutin (antibiotics for infections)
- abrocitinib (used to treat atopic dermatitis, also known as atopic eczema)
- alfentanil, fentanyl (used as anesthetics)
- amitriptyline, nortriptyline (used as antidepressants)
- amphotericin B, voriconazole (antifungals)
- medicines that make the blood less viscous, to prevent the formation of clots (warfarin or similar medicines)
- benzodiazepines (midazolam, triazolam or similar medicines) used to help sleep or for anxiety
- carbamazepine, phenytoin (used to treat seizures)
- nifedipine, isradipine, amlodipine, verapamil, felodipine and losartan (for hypertension - high blood pressure)
- olaparib (used for ovarian cancer)
- cyclosporine, everolimus, sirolimus or tacrolimus (to prevent transplant rejection)
- cyclophosphamide, vinca alkaloids (vincristine, vinblastine or similar medicines) used to treat cancer
- halofantrine (used to treat malaria)
- statins (atorvastatin, simvastatin and fluvastatin or similar medicines) used to reduce high cholesterol levels
- methadone (used for pain)
- celecoxib, flurbiprofen, naproxen, ibuprofen, lornoxicam, meloxicam, diclofenac (Non-Steroidal Anti-Inflammatory Drugs (NSAIDs))
- oral contraceptives
- prednisone (steroid)
- zidovudine, also known as AZT; saquinavir (used in patients infected with HIV)
- medicines for diabetes, such as chlorpropamide, glibenclamide, glipizide or tolbutamide
- theophylline (used to control asthma)
- tofacitinib (used for rheumatoid arthritis)
- tolvaptan (used to treat hyponatremia [low sodium levels in the blood] or to slow down kidney function deterioration)
- vitamin A (nutritional supplement)
- ivacaftor (alone or in combination with medicines used for cystic fibrosis)
- amiodarone (used to treat irregular heartbeats, 'arrhythmias')
- hydrochlorothiazide (a diuretic)
- ibrutinib (used to treat blood cancer)
- lurasidone (used to treat schizophrenia).
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines.
Taking Fluconazole Aurovitas with food, drinks and alcohol
You can take the medicine with or without food.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
If you are planning to become pregnant, it is recommended that you wait one week after a single dose of fluconazole before becoming pregnant.
For longer fluconazole treatment cycles, consult your doctor about the need to use adequate contraceptive methods during treatment, which should be continued for one week after the last dose.
Do not take Fluconazole Aurovitas if you are pregnant, think you may be pregnant or are planning to have a baby, or are breast-feeding, unless your doctor has told you to. If you become pregnant while taking this medicine or within 1 week of the last dose, consult your doctor.
Fluconazole taken during the first or second trimester of pregnancy may increase the risk of spontaneous abortion. Fluconazole taken during the first trimester may increase the risk of a baby being born with congenital anomalies affecting the heart, bones and/or muscles.
There have been reports of babies born with congenital anomalies affecting the skull, ears and bones of the thigh and elbow in women treated for three months or more with high doses (400-800 mg daily) of fluconazole for coccidioidomycosis. The relationship between fluconazole and these cases is unclear.
You can continue breast-feeding after taking a single dose of 150 mg of this medicine.
Do not continue breast-feeding if you are taking repeated doses of this medicine.
Driving and using machines
When driving or operating machinery, you should take into account that dizziness or seizures may occasionally occur.
Fluconazole Aurovitas contains lactose monohydrate,a type of sugar.If your doctor has told you that you have an intolerance to some sugars, consult with them before taking this medicine.
Fluconazole Aurovitas contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per hard capsule, which is essentially “sodium-free”.
3. How to take Fluconazole Aurovitas
Follow exactly the instructions of use of this medicine indicated by your doctor or pharmacist.
In case of doubt, consult your doctor or pharmacist again.
The capsules should be swallowed whole with the help of a glass of water. It is best to take your capsules always at the same time of day.
Instructions for removing the capsule from the blister cavity:
Pressing the capsule cavity in the central part may cause deformations/breakages of the capsule as shown in figure A. To avoid such damage to the capsule, press the capsule cavity at one end to remove it from the blister as shown in figure B.
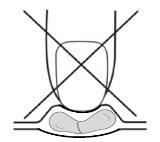
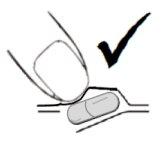
Fig. AFig. B
The following is the recommended dose of this medicine for the different types of infections:
Adults
Condition | Dose |
To treat cryptococcal meningitis | 400 mg on the first day and then 200 mg to 400 mg once a day for 6 to 8 weeks or longer if necessary. Sometimes the dose is increased to 800 mg |
To prevent the recurrence of cryptococcal meningitis | 200 mg once a day until your doctor tells you to |
To treat coccidioidomycosis | 200 mg to 400 mg once a day from 11 months to 24 months or longer if necessary. Sometimes the dose is increased to 800 mg |
To treat internal fungal infections caused by Candida | 800 mg on the first day and then 400 mg once a day until your doctor tells you to |
To treat mucosal infections that affect the lining of the mouth, throat or associated with dental prostheses. | 200 mg to 400 mg on the first day and then 100 mg to 200 mg once a day until your doctor tells you to |
To treat mucosal candidiasis – the dose depends on where the infection is located | 50 mg to 400 mg once a day for 7 to 30 days until your doctor tells you to |
To prevent the recurrence of mucosal infections that affect the lining of the mouth and throat | 100 mg to 200 mg once a day, or 200 mg 3 times a week, while the risk of developing an infection continues |
To treat genital candidiasis | 150 mg in a single dose |
To reduce the repeated appearance of vaginal candidiasis | 150 mg every 3 days for a total of 3 doses (days 1, 4 and 7) and then once a week for 6 months while the risk of developing an infection continues |
To treat fungal skin or nail infections | Depending on the location of the infection 50 mg once a day, 150 mg once a week, 300 to 400 mg once a week for 1 to 4 weeks (for athlete's foot, up to 6 weeks may be necessary, for nail infections, treatment should continue until a healthy nail grows) |
To prevent the spread of a Candidainfection (if your immune system is weak and not working properly) | 200 mg to 400 mg once a day while the risk of developing an infection continues |
Use in children and adolescents
Adolescents from 12 to 17 years of age
Follow the dose indicated by your doctor (the adult posology or the pediatric posology).
Children up to 11 years
The maximum daily dose for children is 400 mg per day.
The dose will be based on the child's weight in kilograms.
Condition | Daily dose |
Mucosal candidiasis and Candidathroat infections – the dose and duration of treatment depend on the severity of the infection and where it is located. | 3 mg per kg of body weight once a day (on the first day, 6 mg per kg of body weight may be given) |
Cryptococcal meningitis or internal fungal infections caused by Candida | 6 mg to 12 mg per kg of body weight once a day |
To prevent the recurrence of cryptococcal meningitis | 6 mg per kg of body weight once a day |
To prevent the child from contracting a Candidainfection (if their immune system is not working properly) | 3 mg to 12 mg per kg of body weight once a day |
Use in children from 0 to 4 weeks of age
Use in children from 3 to 4 weeks of age:
The same dose as described in the table, but administered once every 2 days. The maximum dose is 12 mg per kg of body weight every 48 hours.
Use in children under 2 weeks of age:
The same dose as described in the table, but administered once every 3 days. The maximum dose is 12 mg per kg of body weight every 72 hours.
Elderly patients
The usual adult dose, unless you have kidney problems.
Patients with kidney problems
Your doctor may change your dose, depending on how well your kidneys are working.
If you take more Fluconazole Aurovitas than you should
Taking too many capsules at once can make you feel unwell. In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount taken. It is recommended to take the medicine package and leaflet to the healthcare professional.
The symptoms of a possible overdose may include hearing, seeing, feeling and thinking things that are not real (hallucinations and paranoid behavior). It may be appropriate to initiate symptomatic treatment (with supportive measures and stomach lavage if necessary).
If you forget to take Fluconazole Aurovitas
Do not take a double dose to make up for forgotten doses. If you have forgotten to take a dose, take it as soon as you remember. If it is almost time for the next dose, do not take the missed dose.
If you have any doubts about the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them. Stop taking Fluconazol Aurovitas and seek immediate medical attention if you notice any of the following symptoms:
- Widespread rash, high body temperature, and enlarged lymph nodes (DRESS syndrome or drug hypersensitivity syndrome).
Some people develop allergic reactions, although severe allergic reactions are rare.If any of the following adverse effects appear, talk to your doctor or pharmacist. This includes possible adverse effects not mentioned in this leaflet. If you suffer from any of the following adverse effects, inform your doctor or pharmacist immediately:
- sudden wheezing, difficulty breathing, or chest tightness
- swelling of the eyelids, face, or lips
- itching all over the body, skin redness, or red spots with itching
- skin rash
- severe skin reactions, such as a rash that causes blisters (this can affect the mouth and tongue)
This medicine may affect your liver. Signs that indicate liver problems include:
- fatigue
- loss of appetite
- vomiting
- yellowing of the skin or the whites of the eyes (jaundice)
If you experience any of these symptoms, stop taking this medicine and inform your doctor immediately.
Other Adverse Effects:
Additionally, if you consider that any of the adverse effects you are suffering from is serious or if you notice any adverse effect not mentioned in this leaflet, inform your doctor or pharmacist.
Common adverse effects (may affect up to 1 in 10 people):
- headache
- stomach upset, diarrhea, discomfort, vomiting
- elevated blood test results indicating liver function
- rash
Uncommon adverse effects (may affect up to 1 in 100 people):
- reduction of red blood cells, which can make your skin pale and cause weakness or difficulty breathing
- decreased appetite
- inability to sleep, feeling of numbness
- seizures, dizziness, feeling of spinning, tingling, pinching, or numbness, changes in taste sensation
- constipation, heavy digestion, gas, dry mouth
- muscle pain
- liver damage and yellowing of the skin and eyes (jaundice)
- hives, blisters (hives), itching, increased sweating
- fatigue, general feeling of discomfort, fever
Rare adverse effects (may affect up to 1 in 1,000 people):
- white blood cells in the blood that help defend against infections and blood cells that help stop bleeding, lower than normal.
- red or purple skin discoloration, which may be due to a low platelet count, other changes in blood cells
- changes in blood tests (high cholesterol, fat levels)
- low potassium levels in the blood
- tremors
- abnormal electrocardiogram (ECG), changes in heart rate or rhythm
- liver function failure
- allergic reactions (sometimes severe), including widespread rash with blisters and skin peeling, severe allergic reactions, swelling of the lips or face
- hair loss
Frequency not known but may occur (cannot be estimated from available data):
- hypersensitivity reaction with skin rash, fever, inflamed glands, increased eosinophils (a type of white blood cell), and inflammation of internal organs (liver, lungs, heart, kidneys, and large intestine) (drug reaction or eruption with eosinophilia and systemic symptoms [DRESS])
Reporting Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect not mentioned in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Fluconazol Aurovitas
Keep out of sight and reach of children.
Do not use this medicine after the expiration date stated on the packaging after CAD. The expiration date is the last day of the month indicated.
No special storage conditions are required.
Medicines should not be disposed of via wastewater or household waste. Deposit the packaging and medicines you no longer need at the SIGRE Point in the pharmacy. Ask your pharmacist how to dispose of the packaging and medicines you no longer need. This way, you will help protect the environment.
6. Package Contents and Additional Information
Composition of Fluconazol Aurovitas
- The active ingredient is fluconazole.
- Each capsule contains 50 mg/ 100 mg/ 150 mg or 200 mg of fluconazole.
- The other ingredients (excipients) are: lactose monohydrate, cornstarch, sodium lauryl sulfate, colloidal anhydrous silica, and magnesium stearate.
The composition of the capsules is titanium dioxide (E171), sodium lauryl sulfate, and gelatin.
The printing ink contains shellac and yellow iron oxide (E172).
Appearance of the Product and Package Contents
Fluconazol Aurovitas 50 mg hard capsules EFG
Hard gelatin capsules, white to off-white opaque, size “4”, filled with white to off-white powder, and printed with the letter “FL” on the white to off-white opaque face and with the number “50” on the white to off-white opaque body with yellow ink.
Fluconazol Aurovitas 100 mg hard capsules EFG
Hard gelatin capsules, white to off-white opaque, size “2”, filled with white to off-white powder, and printed with the letter “FL” on the white to off-white opaque face and with the number “100” on the white to off-white opaque body with yellow ink.
Fluconazol Aurovitas 150 mg hard capsules EFG
Hard gelatin capsules, white to off-white opaque, size “1”, filled with white to off-white powder, and printed with the letter “FL” on the white to off-white opaque face and with the number “150” on the white to off-white opaque body with yellow ink.
Fluconazol Aurovitas 200 mg hard capsules EFG
Hard gelatin capsules, white to off-white opaque, size “0”, filled with white to off-white powder, and printed with the letter “FL” on the white to off-white opaque face and with the number “200” on the white to off-white opaque body with yellow ink.
Fluconazol Aurovitas hard capsules are available in PVC/PVDC-Aluminum blisters
Packaging of 1, 2, 3, 4, 6, 7, 10, 12, 14, 20, 28, 30, 42, 50, 60, 100, and 500 hard capsules
Only some pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder:
Aurovitas Spain, S.A.U.
Avda. De Burgos, 16-D
28036 Madrid
Spain.
Manufacturer:
APL Swift Services (Malta) Ltd
HF26, Hal Far Industrial Estate, Hal Far
Birzebbugia, BBG 3000
Malta
or
Generis Farmaceutica, S.A.
Rua João de Deus, n. o 19, Venda Nova
2700-487, Amadora
Portugal
This medicine is authorized in the Member States of the European Economic Area under the following names:
Belgium: Fluconazol AB 50 mg/100 mg/150 mg/200 mg hard capsules
Luxembourg: Fluconazol AB 50 mg/100 mg/150 mg/200 mg hard capsules
Poland: Fluconazole Aurovitas
Portugal: Fluconazol Ritisca
Spain: Fluconazol Aurovitas 50 mg/100 mg/150 mg/200 mg hard capsules EFG.
Date of the Last Revision of this Leaflet:February 2024
Detailed information about this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es)
- Country of registration
- Average pharmacy price30 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FLUCONAZOLE AUROVITAS 200 mg HARD CAPSULESDosage form: CAPSULE, 150 mg fluconazoleActive substance: fluconazoleManufacturer: Arafarma Group S.A.Prescription requiredDosage form: ORAL SOLUTION/SUSPENSION, 10 mg/mlActive substance: fluconazoleManufacturer: Vinci Farma, S.A.Prescription requiredDosage form: CAPSULE, 100 mgActive substance: fluconazoleManufacturer: Vinci Farma, S.A.Prescription required
Online doctors for FLUCONAZOLE AUROVITAS 200 mg HARD CAPSULES
Discuss questions about FLUCONAZOLE AUROVITAS 200 mg HARD CAPSULES, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















